Temperature value
From Proteopedia
In crystallography, uncertainty in the positions of atoms increases with disorder in the protein crystal. Disorder may have two components, static and dynamic. Resolution represents the average uncertainty for all atoms. In contrast, the temperature value (also called temperature factor or B factor) quantitates the uncertainty for each atom. At typical resolutions for protein crystals (where occupancy cannot be distinguished from B value), a high temperature factor reflects a low empirical electron density for the atom, and vice versa. This is illustrated in Electron Density Maps. Generally, a temperature value of less than 30 Å2 signifies confidence in its position, while a temperature value of greater than 60 Å2 signifies disorder[1].
The temperature value is recorded in the atomic coordinate file. In the PDB file format, it is the last numeric value (columns 61-66) in each ATOM and HETATM record. Coloring by temperature is a common way to visualize the uncertainty for each atom.
PDB files for models determined by cryo-EM often specify values in the temperature/B factor field. However, a 2017 analysis concluded that "the treatment of the atomic displacement (B) factors was meaningless in almost all analyzed cryo-EM models"[2].
Contents |
Definition
The temperature factor (also called the temperature value, B factor, B value, or Debye-Waller factor) is
"a factor that can be applied to the X-ray scattering term for each atom (or for groups of atoms) that describes the degree to which the electron density is spread out. While the theory is that the B-factor indicates the true static or dynamic mobility of an atom, it can also indicate where there are errors in model building."[3]
The B-factor is given[3] by
where Ui2 is the mean square displacement of atom i. As U increases, the B-factor increases and the contribution of the atom to the scattering is decreased. If atoms are incorrectly positioned in the model, their B-factors will tend to be higher than correctly positioned atoms nearby[3]. For a B-factor of 15 Å2, the mean square displacement of an atom from its equilibrium position is approximately 0.44 Å, and approximately 0.87 Å for a B-factor of 60 Å2. For comparison, the van der Waals diameter of a carbon atom is 3.4 Å[4].
Disorder
Static Disorder: Some regions of the molecule may adopt different conformations in different copies of the molecule, each molecule's conformation being relatively stable.
Dynamic Disorder: Some regions of every copy of the molecule may be subject to thermal motion, meaning vibration about the rest position[5]. Large thermal motions cease when the crystal is frozen very rapidly with liquid nitrogen, which is the usual procedure prior to irradiation. Typically, protein crystals are kept well below freezing while being irradiated, although irradiation may warm the crystal permitting some thermal motion. Disorder in a frozen protein crystal may include both static disorder and dynamic disorder, the latter being represented by the "snapshot" of thermal disorder frozen in a moment in time.
Some regions of the molecule may have higher average disorder, and others lower average disorder. Typically, the ends of chains have higher average disorder, and hence their positions are less certain than are residues in the core of a tightly packed domain, where disorder is less.
B-factors and coordinate error
Disorder in a crystal is reflected in lower resolution of the diffraction data, as described above. Disorder in the coordinates based on that data can be modeled by introducing B-factors for each atom. They model atomic positions being displaced (by an average distance that is related to the B-factor) from an average position (given by the coordinates). There is no direct relationship between B-factor and coordinate error. If we obtain perfect information about the electron density at atomic resolution, we can infer the average position of an atom even if it has a high B-factor (this amounts to finding the center of a fuzzy dilute cloud as opposed to a compact dense cloud). However, there is an indirect relationship: Higher disorder in a crystal results in lower resolution of the diffraction data, resulting in high coordinate errors. At the same time, the average B-factor of the model will be high, reflecting the disorder of the crystal, so overall coordinate error and overall B-factor will be correlated. For individual atoms or regions of the structure with higher disorder, there is a higher chance for systematic errors in building the model, so this correlation of high B-factors and high coordinate errors extends to separate regions of the protein as well (see [1], Figure 4 for an example of correlating B-factors with coordinate errors, given specific values for resolution, completeness and free R-factor, which influence coordinate errors as well.)
Coloring by Temperature
|
Visualizing relative disorder or uncertainty in atomic positions is done by coloring by temperature value. Atoms with low temperature values are colored blue, while atoms with high temperature values are colored red. Light blue, white, and pink atoms represent the scale of intermediate temperature values. The temperature values themselves are relative, not absolute, and hence the colors are also relative. The most important information from coloring by temperature is to identify the red residues whose positions are least certain. Their positions should be taken only as rough approximations, and this is especially worth knowing if any residues in areas of special interest are red.
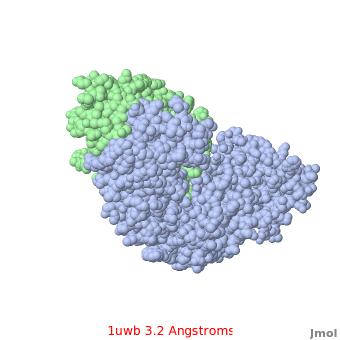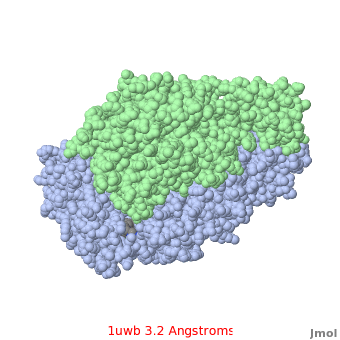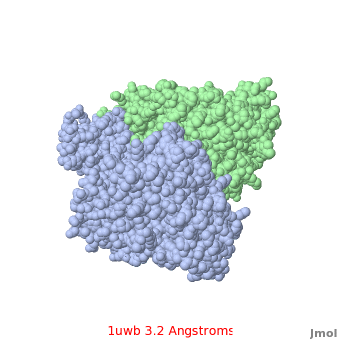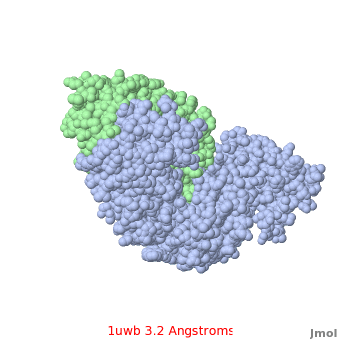
Comparison of the temperature colors of two separate models is risky, since some methodological issues can bias the temperature factors. There are two temperature coloring schemes: absolute and relative. In general, high resolution models tend to have fewer red atoms than do models with modest resolution. For example, a (1kzk) has , while the (1uwb) has . (These scenes are colored by relative temperature. Coloring by absolute temperature looks very similar in these particular cases. See other examples.)
See also Temperature value vs. resolution.
In 1uwb, many of the surface sidechains are missing (due to disorder). Missing sidechains are labeled S- in FirstGlance in Jmol (linked under the molecule on all PDB code-titled pages in Proteopedia).
Most molecular modeling and visualization software packages have an option to color by temperature, including FirstGlance in Jmol, which is linked on every page in Proteopedia that is titled with a PDB code. In FirstGlance in Jmol (version 2), this option will be found under the Views tab, as Local Uncertainty.
Missing Residues and Atoms
Often the very ends of chains, or surface loops, may be so disordered as to prevent assigning any atomic positions at all, leading to missing residues. That is, these residues were present in the crystallized protein, but have no coordinates in the atomic model because their electron densities were too indistinct.
FirstGlance in Jmol (linked beneath the molecule on every PDB code-titled page in Proteopedia) lists missing residues and marks their positions with eye-catching "empty baskets".
The sequence listing for a PDB code offered by PDB-Europe makes it easy to see missing residues: they are highlighted with a gray background. FirstGlance in Jmol has links to these listings under Sequences.
Alternatively, at the PDB, the Sequence tab provides a graphic representation of the sequence that indicates gaps in two ways. First, the thin black line underneath the sequence is broken; second, touching a residue above breaks in the line reports "no identifier from ATOM record (no structural data available)". However, it is easy to overlook breaks in the line.
In addition to entire residues missing from the atomic model, side chains atoms may be missing (due to disorder), even when the main chain atoms are present. FirstGlance in Jmol puts the label S- on every residue with missing side chain atoms.
In the PDB file format, missing residues are listed in REMARK 465, while missing atoms are listed in REMARK 470.
Data Format
In the PDB file format, each atom is given not only X, Y, and Z Cartesian coordinates, but two additional values immediately following called occupancy and temperature value (also known as the isotropic B value, temperature factor, Debye-Waller factor, or B-factor). If the end of a chain adopts either of two stable positions with equal probability, each position has 50% occupancy. The temperature factor is provided to quantitate the level of thermal motion. However, these two components of disorder cannot be distinguished with crystal diffraction data alone. Therefore, the occupancy is often given as 1.0 (100%), while the degree of "blur" in the electron density map, representing both components of disorder, is reported in the temperature value field. These values are mapped to colors when a crystallographic result is colored by temperature.
Uncertainty in NMR Models
NMR models provide no information in the temperature value fields of PDB files. Rather, the variation between the models gives some indication of uncertainty or flexibility.
See Also
- Temperature color schemes
- Temperature value vs. resolution
- Resolution
- Quality assessment for molecular models
- NMR Ensembles of Models
- Anisotropic refinement
- Intrinsically Disordered Protein
References Cited
- ↑ Macromolecular Crystallography at ProXyChem.com
- ↑ Wlodawer A, Li M, Dauter Z. High-Resolution Cryo-EM Maps and Models: A Crystallographer's Perspective. Structure. 2017 Oct 3;25(10):1589-1597.e1. doi: 10.1016/j.str.2017.07.012. Epub, 2017 Aug 31. PMID:28867613 doi:http://dx.doi.org/10.1016/j.str.2017.07.012
- ↑ 3.0 3.1 3.2 This description of the B-factor is quoted from Robert L. Campbell's website on Protein Crystallography at Queens University, Kingston, Ontario, Canada.
- ↑ van der Waals radii in Wikipedia
- ↑ Rhodes, G. 2006. Crystallography Made Crystal Clear, 3rd ed. Academic Press.
Content Donors
The section #B-factors and coordinate error was written by User:Karsten Theis and moved to this article by Eric Martz.
Large portions of the initial version of this page were adapted from the Glossary of ProteinExplorer.Org by the principal author, Eric Martz.