User:Eran Hodis/Sandbox7
From Proteopedia
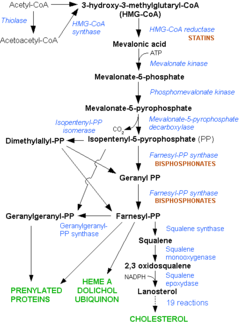
HMG-CoA Reductase (or 3-hydroxy-3-methyl-glutaryl-CoA reductasese or HMGR) is the rate-controlling enzyme of the mevalonate pathway, responsible for cholesterol and other isoprenoid biosynthesis. HMGR is a polytopic, transmembrane protein, containing 8 domains, that is anchored in the membrane of the endoplasmic reticulum.[1] It is the major target of the Statins, a cholesterol lowering drug class and the best selling pharmaceutical drugs in the world.
Contents |
Biological Role
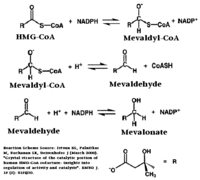
HMGR is among the most highly regulated enzymes in the human body. It catalyzes the formation of mevalonic acid, the committed step in the biosynthesis of sterols, most notably cholesterol. This reaction can be seen below where HMG-CoA is reduced by NADPH. Despite the poor reputation cholesterol has in the media, it is a critical component of cellular membranes as it is required to establish proper membrane permeability and fluidity. The mevalonate pathway is also responsible for synthesis of the oxygen transporting heme found in red blood cells. [2]
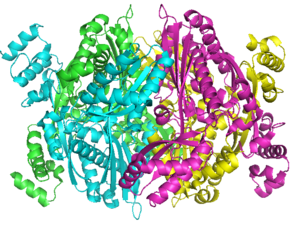
Structure
| |||||||||||
Medical Implications
|
Elevated cholesterol levels have been identified as a major risk factor for coronary artery disease, the narrowing of arteries of the heart, which affected over 13 million people in the United States alone. It is a major cause of disability and death, killing over 500 thousand people in the USA in 2001. [6]
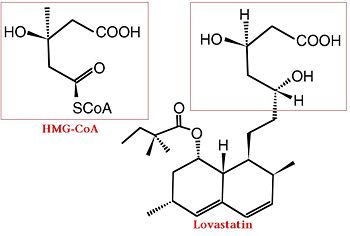
The statins are HMG-CoA reductase inhibitors. Discovered by Akira Endo in 1971, statins are similar in structure to HMG-CoA and act by competitively inhibiting HMGR. Since HMGR is the first committed enzyme in the cascade that eventually produces cholesterol, use of statins can dramatically reduce blood cholesterol levels.[7] As a drug class, statins generated over $20 billion dollars in sales in 2009 with Pfizer’s Lipitor being the best selling drug in the world at the time of writing.[8][9]
A number of crystal structures of HMGR with bound statins have been solved which elucidate how the statin molecule is bound by HMGR. Statins in general occupy the active site of HMGR, preventing HMG-CoA from binding. The structure of HMGR with bound statins () shows that the cis loop forms a number of polar interactions with the statin inhibitor, particularly residues Ser 684, Asp 690, Lys 691, Lys 692, and hydrogen bond interactions between Glu 559 and Asp 767 with the O5-hydroxyl of the statins. Van der Waals interactions between Leu 562, Val 683, Leu 853, Ala 856, and Leu 857 of HMGR and hydrophobic ring structures of the statins contribute to binding as well.[10] These interactions result in the statins binding to HMGR with a Ki of between .1-2.3nM while the Michaelis constant KM for HMG-CoA is 4uM, allowing the statins to outcompete HMG-CoA in binding to HMGR.[11] Additional structures of HMGR with the statins , , , , and highlight the important residues involved in inhibitor binding.
Additional 3D Structures of HMG-CoA Reductase
3cct, 3ccw, 3ccz, 3cd0, 3cd5, 3cd7, 3cda, 3cdb – HMG-CoA Reductase Catalytic Domain
2qil – HMG-CoA Reductase Catalytic Domain
2q6b, 2q6c – Catalytic Domain of HMG-CoA Reductase
1t02 – Crystal Structure of Statin Bound HMG-CoA Reductase
1r31 – Crystal Structure of HMGR from Pseudomonas mevalonii
1hw8, 1hw9, 1hwi, 1hwj, 1hwk, 1hwl – HMG CoA Reductase with Various Statins Bound
1qax, 1qay – Ternary Complex of Pseudomonas Mevalonii HMGR with mevalonate
Additional Resources
- See: Pharmaceutical Drug Targets For Additional Information about Drug Targets for Related Diseases
- See: Metabolic Disorders For Additional Information.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Roitelman J, Olender EH, Bar-Nun S, Dunn WA Jr, Simoni RD. Immunological evidence for eight spans in the membrane domain of 3-hydroxy-3-methylglutaryl coenzyme A reductase: implications for enzyme degradation in the endoplasmic reticulum. J Cell Biol. 1992 Jun;117(5):959-73. PMID:1374417
- ↑ 2.0 2.1 2.2 Meigs TE, Roseman DS, Simoni RD. Regulation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase degradation by the nonsterol mevalonate metabolite farnesol in vivo. J Biol Chem. 1996 Apr 5;271(14):7916-22. PMID:8626470
- ↑ Istvan ES, Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2001 May 11;292(5519):1160-4. PMID:11349148 doi:10.1126/science.1059344
- ↑ Song BL, Sever N, DeBose-Boyd RA. Gp78, a membrane-anchored ubiquitin ligase, associates with Insig-1 and couples sterol-regulated ubiquitination to degradation of HMG CoA reductase. Mol Cell. 2005 Sep 16;19(6):829-40. PMID:16168377 doi:10.1016/j.molcel.2005.08.009
- ↑ Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990 Feb 1;343(6257):425-30. PMID:1967820 doi:http://dx.doi.org/10.1038/343425a0
- ↑ www.nhlbi.nih.gov/health/.../Diseases/.../CAD_WhatIs.html
- ↑ Endo A, Kuroda M, Tanzawa K. Competitive inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase by ML-236A and ML-236B fungal metabolites, having hypocholesterolemic activity. FEBS Lett. 1976 Dec 31;72(2):323-6. PMID:16386050
- ↑ http://www.drugs.com/top200.html
- ↑ http://www.medicalnewstoday.com/articles/25046.php
- ↑ Istvan ES, Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2001 May 11;292(5519):1160-4. PMID:11349148 doi:10.1126/science.1059344
- ↑ Corsini A, Maggi FM, Catapano AL. Pharmacology of competitive inhibitors of HMG-CoA reductase. Pharmacol Res. 1995 Jan;31(1):9-27. PMID:7784310