Sandbox Reserved 403
From Proteopedia
| This Sandbox is Reserved from September 14, 2021, through May 31, 2022, for use in the class Introduction to Biochemistry taught by User:John Means at the University of Rio Grande, Rio Grande, OH, USA. This reservation includes 5 reserved sandboxes (Sandbox Reserved 1590 through Sandbox Reserved 1594). |
To get started:
More help: Help:Editing. For an example of a student Proteopedia page, please see Photosystem II, Tetanospasmin, or Guanine riboswitch. |
| |||||||
|
Contents |
Tetanospasmin (Tetanus Neurotoxin)(TeNT)
Clostidium tetani
The Gram positive bacilli Clostridium tetani is the bacteria responsible for the disease state of tetanus. The presence of the bacteria does not cause the disease but instead the toxins it produces cause the disease state. C. tetani produces two toxins; tetanospasmin and tetanolysin. Tetanolysin is a cytolysin that increases the permeability of cellular membranes through cell lysis.[1] Tetanospasmin is the cause of tetanus and is sometimes referred to as tetanus neurotoxin (TeNT), as it acts on the central nervous system. Tetanospasmin makes its way to the central nervous system via retrograde axonal flow beginning with α- motor neurons found in muscle and ending by binding to gangliosides found in the central nervous system (CNS).[2]

Retrograde Axonal Transport
After internalization into the α-motor neuron membrane TeNT is transported via retrograde axonal transport. Retrograde axonal transport is a normal process within the cell membranes of neurons that allows them to remove and recycle cellular debris from axons. Two organelles have been identified as retrograde carriers within axons: round vesicles and tubulo-vesicular structures. These structures work to protect TeNT from lysosomal degradation and acidification, delivering it to the inhibitory interneurons of the CNS in a fully active form. These particular organelles may attach TeNT by a neutrophin receptor p75 (p75NTR), which is used in the retrograde transport Nerve Growth Factor (NGF).[4]
Gangliosides
Gangliosides are in the category of glycosphingolipids and are found predominately in neuronal tissues. Gangliosides consist of sialic acid linked to a sugar (glucose, galactose, GalNAc, GlcNAc and/or fructose) backbone attached to a ceramide base. These gangliosides make up approximately 10% of a neuron’s total lipid content and like other lipids, gangliosides function in cell signal transduction.[5]
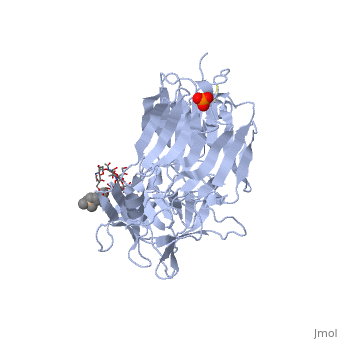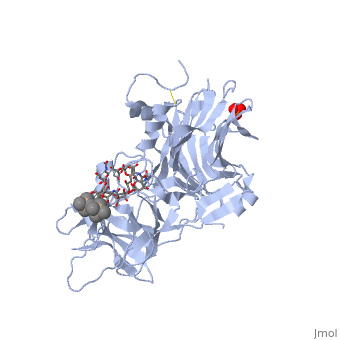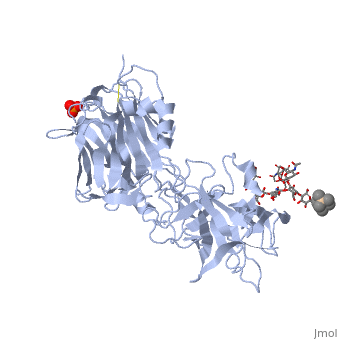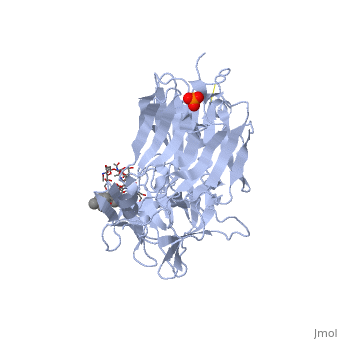
Tetanospasmin (TeNT)
Tetanospasmin is a 150-kDa toxin that is composed of one light chain (50-kDa) and one heavy chain (100-kDa). The light chain is responsible for the toxicity of the molecule, whereas the heavy chain is responsible for binding the toxin to the axonal membranes. The heavy chain can also be cleaved into 2 fragments Hn and Hc. The Hn fragment is responsible for the translocation of the light chain across the axonal membrane, whereas the Hc fragment binds to the axonal membrane.[6]
Hc and Ganglioside Interaction

Hc has two distinct domains:[7]

1. Jelly-roll (amino end)
2. β-Trefoil (carboxyl end)
Studies have shown that the β-trefoil domain contains the ganglioside binding sites.[7]
Binding studies have shown that a particular ganglioside, GT1-b, is necessary for the binding of the Hc fragment of tetanospasmin (TeNT). An analogue of the GT1-b ganglioside was made in order to increase solubility because a crystal structure of the Hc and native GT1-b could not be obtained.

The Hc fragment has two binding sites in the β-trefoil domain:[7]
At this site a narrow groove is formed where a number of hydrogen bonds can form.[7]
Common hydrogen bonds are formed between the side chain of His1271 and OH-6, OH-4 and O-5 of Gal4 and between the main chain carbonyl oxygen of Thr1270 and OH-4 of Gal4. GalNAc3 interacts via a hydrogen bond between OH-4 andAsp1222 OD and between OH-4 and His1271. Ring stacking involving galactose also occurs in this site.
At this site a shallow pocket is formed where hydrogen bonding occurs.[7]
Commonly hydrogen bonds form between OD-1 and OD-2 of Asp1147 and O-4 and the acetamido-N-5 of Sia6 and between ND-2 of Asn1216 and O-10 of Sia6. A salt bridge also forms between Arg1226 and the sialic acid of Sia7, also the carboxylate group and hydrogen bonds between O-1A and the amide NH of Asn1216; between O-4 and the carbonyl oxygen of Asp1214; and betweenOH-8 and Tyr1229 hydroxyl group on Sia7.
Modeling suggests that it is possible for the two arms of the ganglioside to interact with more than one Hc fragment. This may result in clustering and crosslinking of the toxin and enhance the process internalization or uptake of the toxin through the axonal membrane.[7] Others suggest that TeNT can directly cross link two gangliosides through its single protein domain which also enhances the uptake of the toxin.[6] Therefore by binding at one or both of the sites, the Hc fragment of tetanospasmin is successfully able to assist the rest of the tetanospasmin molecule in gaining access to the cytoplasm of the inhibitory interneurons.
References
- ↑ Hatheway CL. Toxigenic clostridia. Clin Microbiol Rev. 1990 Jan;3(1):66-98. PMID:2404569
- ↑ Bizzini B. Tetanus Toxin. Microbiological Reviews.1979 June;43(2):224-236.[1]
- ↑ Mechanism of Action of Tetanospasmin (Dr. Arnab K Rana) [image on the internet]. 2005[updated 2005 Dec 26; cited 2011 Apr 20]. Available from: http://en.wikipedia.org/wiki/File:Mechanism_of_action_of_tetanospasmin.gif
- ↑ Lalli G, Schiavo G. Analysis of retrograde transport in motor neurons reveals common endocytic carriers for tetanus toxin and neurotrophin receptor p75NTR. J Cell Biol. 2002 Jan 21;156(2):233-9. Epub 2002 Jan 21. PMID:11807088 doi:10.1083/jcb.200106142
- ↑ Mocchetti I. Exogenous gangliosides, neuronal plasticity and repair, and the neurotrophins. Cell Mol Life Sci. 2005 Oct;62(19-20):2283-94. PMID:16158191 doi:10.1007/s00018-005-5188-y
- ↑ 6.0 6.1 Chen C, Fu Z, Kim JJ, Barbieri JT, Baldwin MR. Gangliosides as high affinity receptors for tetanus neurotoxin. J Biol Chem. 2009 Sep 25;284(39):26569-77. Epub 2009 Jul 14. PMID:19602728 doi:10.1074/jbc.M109.027391
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 7.8 Fotinou C, Emsley P, Black I, Ando H, Ishida H, Kiso M, Sinha KA, Fairweather NF, Isaacs NW. The crystal structure of tetanus toxin Hc fragment complexed with a synthetic GT1b analogue suggests cross-linking between ganglioside receptors and the toxin. J Biol Chem. 2001 Aug 24;276(34):32274-81. Epub 2001 Jun 19. PMID:11418600 doi:10.1074/jbc.M103285200