Anthony Noles Sandbox
From Proteopedia
Contents |
Aconitase
Aconitase (PDB 7acn) is a single polypeptide (Mr 83kD) that catalyzes the reversible isomerization of citrate and isocitrate.[1] It is the second enzyme in the Citric acid cycle, which is a series of enzyme-catalysed chemical reactions that is crucial to aerobic cellular respiration and the production of ATP.
Structure
The consists of numerous alternating alpha helices and beta sheets (SCOP classification α/β alternating). The tertiary structure is somewhat bilobed with the active site in the middle, and, since there is only one subunit, there is no quaternary structure. Aconitase consists of four domains, three of which are tightly packed while the fourth is more flexible. [2] Aconitase contains a . This iron sulfur cluster does not participate in redox as most do, but holds the OH group of citrate to facilitate its elimination.[3] It is at this 4Fe-4S site that catalysis occurs and citrate or is bound. The rest of the is made up of residues Gln72, Asp100, His101, Asp165, Ser166, His167, His147, Glu262, Asn258, Cys358, Cys421, Cys424, Cys358, Cys421, Asn446, Arg447, Arg452, Asp568, Ser642, Ser643, Arg644, Arg580. [4]
| |||||||||
| 7acn, resolution 2.00Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , | ||||||||
| Non-Standard Residues: | |||||||||
| Activity: | Aconitate hydratase, with EC number 4.2.1.3 | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
Mechanism of Aconitase
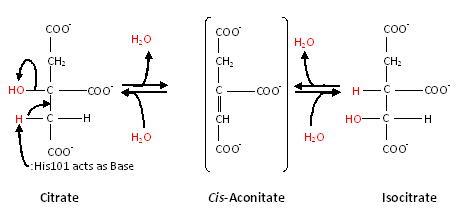
Substrate-free aconitase contains a [4Fe-4S]2+ cluster with hydroxyl bound to one of the Fe. Upon binding of substrate the bound hydroxyl is protonated. A hydrogen bond from to the isocitrate hydroxyl is donated to form water. Alternatively, the proton could be donated by as this histidine is hydrogen bonded to a H2O molecule. His167 is also hydrogen bonded to the bound H2O in the [4Fe-4S] cluster. Both are paired with carboxylates (, respectively) and are likely to be protonated. The conformational change associated with substrate binding reorients the cluster. [4] The residue which removes a proton from citrate or isocitrate is . [4] This causes the cis-Aconitate intermediate (seen below), which consists of a double bond, which is a direct result of the deprotonation. Then, there is a rehydration of the double bond of cis-aconitate to form isocitrate (if the original substrate was citrate). To better understand this, consider this process as stages, seen below.
Stage 1: Dehydration
First, dehydration of citrate causes a proton and OH group to be removed from only the 'lower arm'.[5] This forms a cis-Aconitate intermediate.
Stage 2: Rehydration
The second main stage of the reaction is the rehydration of the cis-Aconitate intermediate. This forms isocitrate. It is catalyzed in a stereospecific way such that only one isocitrate stereoisomer is formed. [5]
Thus, the overall reaction that aconitase catalyzes is: Citrate ←→cis-Aconitate←→Isocitrate, as seen below:
Regulation
Aconitase can be inhibited or activated to increase or decrease the ability to catalyze the reaction of citrate to isocitrate. The activity of aconitase can be reduced when one Fe is lost from the cluster. This lowers the activity over 100-fold, but then can regain full activity by adding another Fe from solution. [6] Aconitase is also strongly inhibited by nitro analogs [6]
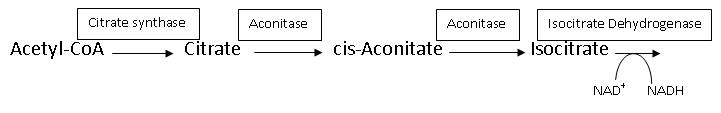
The Citric Acid Cycle works in such a way that the product of one reaction becomes the reactant of another, with different enzymes catalyzing each reaction. Aconitase is one such enzyme. Some of these enzymes are tightly regulated, either activated or inhibited, by the concentration of reactant, product, ATP or NADH, and thus are rate-determining. Aconitase is not one of the three rate-determining enzymes of the Citric Acid Cycle as its ΔG is not negative (ΔG°′≈5 kJ/mol and ΔG≈0 kJ/mol).[5] Aconitase functions close to equilibrium and the rate of citrate consumption depends on the activity of NAD+-dependent isocitrate dehydrogenase, which is one of the three rate-determining enyzmes. Isocitrate dehydrogenase uses the product of the reaction aconitase catalyzes. Both Citrate synthase and Isocitrate dehydogenase are inhibited by NADH concentration, but aconitase itself is not.[5] Since the rate of aconitase depends on the activity of NAD+-dependent isocitrate dehydrogenase, then citrate could build up on the reactant side, which would then inhibit the enzyme of the previous step, citrate synthase. An illustration of this is seen below, with the boxes representing the enzymes that are catalyzing each reaction. This is a common example of how the Citric Acid Cycle works in order to produce ATP without wasting resources. Similar inhibition/activation of enzymes occurs based on concentrations of ATP, NADH, Calcium, CoA, and others.
Other Functions
Along with serving as a catalyst, aconitase is a member of the iron regulatory protien-1 (IRP-1) family. These enzymes have been found to play a role in regulatory RNA-binding proteins. This suggests a novel role for Fe-S clusters as post-translational regulatory switches.[2]
References
- ↑ Zheng, L., Kennedy, MC., Beinert, H., Zalkin, H. "Mutational analysis of active site residues in pig heart aconitase." J Biol Chem 1992, 267, 7895-7903.
- ↑ 2.0 2.1 Frishman, D., and Hentze, M.W., "Conservation of aconitase residues revealed by multiple sequence analysis: Implications for structure/function relationships." European Journal of Biochemistry, 1996, 239, 197-200.
- ↑ Dupuy J, Volbeda A, Carpentier P, Darnault C, Moulis JM, Fontecilla-Camps JC. Crystal structure of human iron regulatory protein 1 as cytosolic aconitase. Structure. 2006 Jan;14(1):129-39. PMID:16407072 doi:10.1016/j.str.2005.09.009
- ↑ 4.0 4.1 4.2 Beinert, H., Kennedy, M. C., Stout, C.D. “Aconitase as Iron−Sulfur Protein, Enzyme, and Iron-Regulatory Protein.” Chem. Rev. 1996, 96, 2335−2373.
- ↑ 5.0 5.1 5.2 5.3 Voet, Donald, Judith G. Voet, and Charlotte W. Pratt. Fundamentals of Biochemistry Life at the Molecular Level. New York: John Wiley & Sons, 2008. p. 578-579. Print.
- ↑ 6.0 6.1 Flint, DH., and Allen, RM. "Iron-sulfur protein with nonredox functions.” Chem. Rev. 1996, 96, 2315−2334.

