User:Madelyn Kasprzak/Sandbox 92021
From Proteopedia
Structure and Function
The human tau protein, encoded by chromosome 17q21, has a natively unfolded protein structure, which contributes to its flexibility and ability to stabilize functional microtubules [1]. Specifically, its primary structure, consisting of , is highly hydrophilic compared to other cytosolic proteins [1]. It has a predominantly acidic N-terminal region, a proline-rich middle region, and a relatively neutral C-terminal region [1].
|
Additionally, tau has a transient secondary structure of α-helices, β-pleated sheets, and a poly-proline II helix [1]. Tau does not resemble a globular protein, but has characteristics of a denatured, unfolded protein which contributes to its overall hydrophilicity [2]. It can also interact with other tau proteins to form .
Alternative Splicing
Alternative splicing of the human tau gene 17q21 generates several isoform structures, which differ by one or two small inserts in its N-terminal region [3]. Although there are 16 exons within the 17q21 gene, exons 4A, 6, and 8 are expressed in peripheral rather than neural tau proteins [4]. Alternatively, eight of the exons (1, 4, 5, 7, 9, 11, 12, and 13) are involved in structural aspects of tau [4]. Alternative splicing of exons 2, 3, and 10 is responsible for the 6 isoforms of tau [3]. These isoforms differ in 3 or 4 tubulin binding domains within the C-terminal region [5].
Chemical Alterations Tau proteins typically undergo post-translational modifications such as O-glycosylation and phosphorylation. O-glycosylation involves the addition of an O-linked N-acetylglucosamine residue on a serine or threonine amino acid adjacent to the proline-rich domain [4]. This modification is not fully understood, however it may be involved in interactions with tubulin and degradation of tau proteins [4]. Several phosphorylation sites have been identified on mainly serine-proline and threonine-proline amino motifs [4]. Tau protein kinases typically catalyze the phosphorylation of tau; however, non-proline directed kinases could also phosphorylate other sites within tau [4]. Tau phosphorylation directly affects its ability to bind to microtubules [4], as described below.
Tau's Role in Microtubules and Axonal Transport Tau binds to microtubules and aids in their assembly and stabilization as well as the motor-driven transport of axons [6]. Its ability to stabilize microtubules is attributed to repetitive microtubule-binding motifs within its C-terminal region [3]. These 18-amino acid repeat domains bind to microtubules through weak interactions such as van der Waals or ionic bonds [7]. Specifically, the area in the interregion between repeats 1 and 2 is highly potent in microtubule polymerization [5]. Although the repeat domains are similar in structure, they differ in their binding affinities, which is likely attributed to slight differences in their amino acid sequence [7]. Their flexibility and lack of binding cooperativity further accentuate the protein’s transient secondary structure [7].
The phosphorylation state of tau directly affects proper assembly and stabilization of microtubules [5]. Phosphorylation is greatly increased when mutations of the tau protein are present [8]. Phosphate groups on a highly phosphorylated tau may disrupt non-covalent interactions between the protein and microtubules, which may reduce tubulin stability and impair axonal transport. Thus, any abnormalities in the protein’s phosphorylation state may contribute to neurodegeneration, as described below [9].
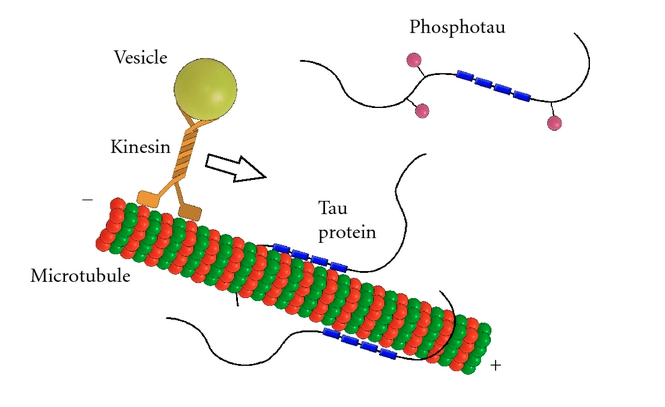
Disease Relation
Alzheimer’s Disease and Dementia A common abnormality in tau phosphorylation, associated with Alzheimer’s disease, is the phosphorylation of two serine-proline motifs located 199 or 202 amino acids upstream of the microtubule-binding region [10]. Phosphorylated helical filaments within these motifs can lead to neurofibrillary tangles, also known as aggregations of tau proteins [2]. Lysine acetylation catalyzed by tau autoacetyltransferase activity can also lead to tau aggregation, thereby preventing tau from interacting with microtubules [11]. Dysfunction of the neuronal cell, likely caused by tau aggregation, can lead to gradual but steady neurodegeneration, which is correlated with the onset of dementia [12]. Tau aggregation may contribute to neuronal loss via cell death in areas of the brain responsible for memory and learning [5].
Parkinson’s Disease Protein aggregation also exists in patients suffering from Parkinson’s disease, specifically within the substantia nigra of the midbrain [13]. Immunohistochemical studies reveal that abnormally phosphorylated tau proteins are partially involved in the development of Lewy bodies, protein aggregates, within the substantia nigra [14]. Lewy bodies may be involved in the neurodegeneration of dopaminergic neurons within this area [13].
References
- ↑ 1.0 1.1 1.2 1.3 Mandelkow EM, Mandelkow E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb Perspect Med. 2012 Jul;2(7):a006247. doi:, 10.1101/cshperspect.a006247. PMID:22762014 doi:http://dx.doi.org/10.1101/cshperspect.a006247
- ↑ 2.0 2.1 Schweers O, Schonbrunn-Hanebeck E, Marx A, Mandelkow E. Structural studies of tau protein and Alzheimer paired helical filaments show no evidence for beta-structure. J Biol Chem. 1994 Sep 30;269(39):24290-7. PMID:7929085
- ↑ 3.0 3.1 3.2 Lei P, Ayton S, Finkelstein DI, Adlard PA, Masters CL, Bush AI. Tau protein: relevance to Parkinson's disease. Int J Biochem Cell Biol. 2010 Nov;42(11):1775-8. doi:, 10.1016/j.biocel.2010.07.016. Epub 2010 Aug 1. PMID:20678581 doi:http://dx.doi.org/10.1016/j.biocel.2010.07.016
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 Buee, L, Bussiere, T, Buee-Scherrer, V, Delacourte, A, Hof, PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res. Rev. 33:95-130 (2000). DOI: 10.1016/S0165-0173(00)00019-9
- ↑ 5.0 5.1 5.2 5.3 5.4 Kolarova M, Garcia-Sierra F, Bartos A, Ricny J, Ripova D. Structure and pathology of tau protein in Alzheimer disease. Int J Alzheimers Dis. 2012;2012:731526. doi: 10.1155/2012/731526. Epub 2012 May, 29. PMID:22690349 doi:http://dx.doi.org/10.1155/2012/731526
- ↑ Qurashi I, Collins J, Chaudhry I, Husain N. Promising use of minocycline augmentation with clozapine in treatment-resistant schizophrenia. J Psychopharmacol. 2014 Mar 19;28(7):707-708. PMID:24646811 doi:http://dx.doi.org/10.1177/0269881114527358
- ↑ 7.0 7.1 7.2 Butner KA, Kirschner MW. Tau protein binds to microtubules through a flexible array of distributed weak sites. J Cell Biol. 1991 Nov;115(3):717-30. PMID:1918161
- ↑ Alonso Adel C, Mederlyova A, Novak M, Grundke-Iqbal I, Iqbal K. Promotion of hyperphosphorylation by frontotemporal dementia tau mutations. J Biol Chem. 2004 Aug 13;279(33):34873-81. Epub 2004 Jun 9. PMID:15190058 doi:http://dx.doi.org/10.1074/jbc.M405131200
- ↑ Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004 Jul;10 Suppl:S10-7. PMID:15272267 doi:http://dx.doi.org/10.1038/nm1066
- ↑ Biernat J, Mandelkow EM, Schroter C, Lichtenberg-Kraag B, Steiner B, Berling B, Meyer H, Mercken M, Vandermeeren A, Goedert M, et al.. The switch of tau protein to an Alzheimer-like state includes the phosphorylation of two serine-proline motifs upstream of the microtubule binding region. EMBO J. 1992 Apr;11(4):1593-7. PMID:1563356
- ↑ Cohen TJ, Friedmann D, Hwang AW, Marmorstein R, Lee VM. The microtubule-associated tau protein has intrinsic acetyltransferase activity. Nat Struct Mol Biol. 2013 Jun;20(6):756-62. doi: 10.1038/nsmb.2555. Epub 2013 Apr, 28. PMID:23624859 doi:http://dx.doi.org/10.1038/nsmb.2555
- ↑ Kadavath H, Hofele RV, Biernat J, Kumar S, Tepper K, Urlaub H, Mandelkow E, Zweckstetter M. Tau stabilizes microtubules by binding at the interface between tubulin heterodimers. Proc Natl Acad Sci U S A. 2015 Jun 16;112(24):7501-6. doi:, 10.1073/pnas.1504081112. Epub 2015 Jun 1. PMID:26034266 doi:http://dx.doi.org/10.1073/pnas.1504081112
- ↑ 13.0 13.1 Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004 Jul;10 Suppl:S10-7. PMID:15272267 doi:http://dx.doi.org/10.1038/nm1066
- ↑ Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW. Colocalization of tau and alpha-synuclein epitopes in Lewy bodies. J Neuropathol Exp Neurol. 2003 Apr;62(4):389-97. PMID:12722831