User:Brittany Carroll/Sandbox1
From Proteopedia
Contents |
tRNA(His) guanylyltransferase
| |||||||||||
Introduction
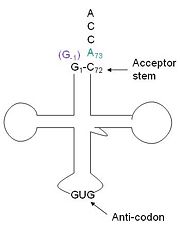
Transfer RNA (tRNA) is the direct connection of nucleic acid sequences and amino acid sequences. tRNA is an adaptor protein that carries the amino acid to the messenger RNA. To prevent potentially detrimental mutations in proteins it is imperative that the correct amino acid gets attached to the proper tRNA. There are a couple way s in which this is regulated, first there is a specific residue or set of residues that are necessary and sufficient for aminoacylation. Secondly, each tRNA has a unique tRNA synthatase, the enzyme that catalyzes aminoacylation. Generally, the terminal residue of the acceptor stem (usually residue 73), and or the anticodon , specifically the middle base 35. A unique amino acid, hisitidine, requires a unique tRNA. This may be because improper insertion or deletion of His could be deleterious to a protein, given the unique properties of His. tRNAHis has a guanine monophosphate (GMP) residue at the 5’ end in all domains of life, besides α-proteopbacteria. This GMP is referred to as G-1. In prokaryotes G-1 is encoded in the genome. RNase P cleaves pre-tRNAHis to generate the mature tRNA, leaving an extra basepair on the acceptor stem, G-1:C73. In eukaryotes the G-1 residue is not encoded and needs to be added post-transcription. The enzyme that catalyzes this reaction is the polymerase,tRNAHis guanylyltransferase (Thg1). Howerver, the addition of the GMP residue is nontemplated, inserting GMP across from A73 in the acceptor stem creating a mismatch. Unlike most polymerases, Thg1 adds nucleotides in the 3’ –to- 5’ direction, while forming a normal 3’ –to- 5’ phosphodiester bond. Therefore, the 3’-OH of the incoming nucleotide attacks the 5’ end of the polynucleotide chain. This is a three step mechanism where the polynucleotide chain is first adenylated (5’ to 5’ linkage) and then guanylated (3’ to 5’ bond) by Thg1. Then a phosphatase removes the two phosphates yielding a monophosphorylated tRNA. Although G-1 is encoded in bacterial and archeal genomes they still have Thg1-like proteins (TLPs). TLPs are used in proofreading and editing tRNA species; they do not appear to be selective for only for tRNA His.[1][2][3][4][5]
This addition is interesting for multiple reasons. It is one of only a few known reactions where a normal 3’-to-5’ phosphodiester bond is formed in a 3’ –to- 5’ direction. Also, the additional 5’ nucleotide is unique to tRNAHis, with the exception of a tRNAPhe species. Lastly, this modification is essential, at least in yeast.[4] The first crystal structure of Thg1 was the 2.3Å human Thg1 solved in 2010.[5]
Homology
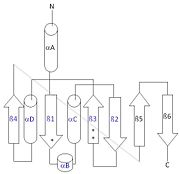
Structure
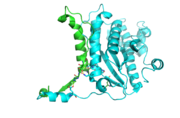
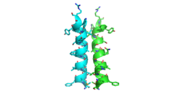
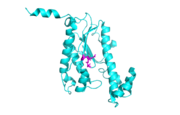
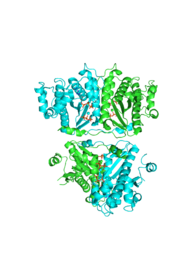
Each monomer has an antiparallel β-sheet with six strands and seven α-helices around the sheet. Thg1 forms a homotetramer, with extensive contacts between the . Even though there are fewer contacts between the dimer of dimers, the tetramer is the most stable oligomeric form. The dimer is stabilized mainly by hydrogen bonds from αD and β4. There are also two salt bridges that help hold the dimer together: Lys to Asp and Glu to Arg (chain A to B). [7]
The reason for the tetramer remained elusive until the "Candida albicans" Thg1 with tRNA His was solved. The model showed that two tRNA His molecules are coordinated between the , each interacting with three monomers. The acceptor stem of the tRNA is coordinated by the dimer of monomers, and the anitcodon is recognized by the second dimer. The binding to the third subunit, is necessary for recognition but may also aid in the correct positioning for guanylylation. Thg1 is selective to binding tRNA His through the recognition of the anticodon loop with base stacking and hydrogen bonding. G34 is recognized via aromatic stacking, between Phe194 and G37(tRNA), this interaction is not base specific but it is purine specific. Asn202 coordinates U35 through hydrogen bonding and His154 base stacks with the third base G36. These interactions cause distortion of the loop that is stabilized by hydrogen bonds from other crucial surrounding amino acids. The structure with tRNA also lends insight into why Thg1 works in is reverse. It appears that Thg1 is a mirror image of forward polymerases, suggesting that the direction in which the template approaches the active site is important in the direction of addition.[8]
Structural highlights
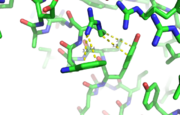
N-terminal helix cap
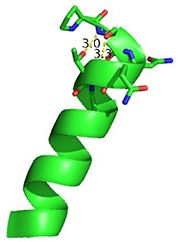
The N-terminal cap of the helix follows a Ib motif. This motif is also known as a capping box.
N’ -> N4 h-xpxph N’- P S N Q T L –N4 (residues 135-140 chain A 3otb)
Hydrogen bonds between N’ and the backbone of N3 and N3 with N’ backbone are shown in the figure. The figure is difficult to see the T with P bb but it is not linear, this may just be due to modeling as it is close enough to form a h-bond. There is also a hydrophobic interaction between P and L.
A cation-π interaction occurs between a cation and the face of a simple aromatic, there is partial negative charge in the center of the ring. The cation-π interaction is actually stronger than a salt bridge because of the desolvation penalty. With the cation-π interaction the cation has a similar dosolvation penalty to pay as the salt bridge ions but the π system is already poorly solvated. Also there is not neutralization of charge that occurs between the two groups. These properties of the cation-π interaction imply that thecation-π interactions on protein surfaces (mainly where they are seen) could contribute to protein structure and stability.
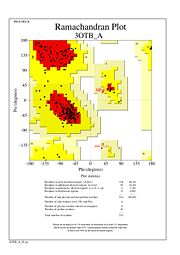
The Ramachandran plot shows that most of the amino acids follow Ramachadran's restraints. The three that are questionable, N32, D67, S75 are all located in turns.
Additional 3D Structures of Thg1
3otc, 3otd, 3ote - Thg1 - Homo sapiens
4kgk, 4kgm - Thg1-like - Bacillus thuringiensis
3wbz, 3wc0, 3wc1, 3wc2 - Thg1 - Candida albicans
References
- ↑ Jackman JE, Gott JM, Gray MW. Doing it in reverse: 3'-to-5' polymerization by the Thg1 superfamily. RNA. 2012 May;18(5):886-99. doi: 10.1261/rna.032300.112. Epub 2012 Mar 28. PMID:22456265 doi:http://dx.doi.org/10.1261/rna.032300.112
- ↑ Hyde SJ, Eckenroth BE, Smith BA, Eberley WA, Heintz NH, Jackman JE, Doublie S. tRNAHis guanylyltransferase (THG1), a unique 3'-5' nucleotidyl transferase, shares unexpected structural homology with canonical 5'-3' DNA polymerases. Proc Natl Acad Sci U S A. 2010 Nov 8. PMID:21059936 doi:10.1073/pnas.1010436107
- ↑ Hyde SJ, Rao BS, Eckenroth BE, Jackman JE, Doublie S. Structural Studies of a Bacterial tRNA(HIS) Guanylyltransferase (Thg1)-Like Protein, with Nucleotide in the Activation and Nucleotidyl Transfer Sites. PLoS One. 2013 Jul 3;8(7):e67465. doi: 10.1371/journal.pone.0067465. Print 2013. PMID:23844012 doi:10.1371/journal.pone.0067465
- ↑ Jackman JE, Phizicky EM. tRNAHis guanylyltransferase adds G-1 to the 5' end of tRNAHis by recognition of the anticodon, one of several features unexpectedly shared with tRNA synthetases. RNA. 2006 Jun;12(6):1007-14. Epub 2006 Apr 19. PMID:16625026 doi:http://dx.doi.org/10.1261/rna.54706
- ↑ Rao BS, Mohammad F, Gray MW, Jackman JE. Absence of a universal element for tRNAHis identity in Acanthamoeba castellanii. Nucleic Acids Res. 2013 Feb 1;41(3):1885-94. doi: 10.1093/nar/gks1242. Epub 2012 , Dec 14. PMID:23241387 doi:http://dx.doi.org/10.1093/nar/gks1242
- ↑ Hyde SJ, Eckenroth BE, Smith BA, Eberley WA, Heintz NH, Jackman JE, Doublie S. tRNAHis guanylyltransferase (THG1), a unique 3'-5' nucleotidyl transferase, shares unexpected structural homology with canonical 5'-3' DNA polymerases. Proc Natl Acad Sci U S A. 2010 Nov 8. PMID:21059936 doi:10.1073/pnas.1010436107
- ↑ Hyde SJ, Eckenroth BE, Smith BA, Eberley WA, Heintz NH, Jackman JE, Doublie S. tRNAHis guanylyltransferase (THG1), a unique 3'-5' nucleotidyl transferase, shares unexpected structural homology with canonical 5'-3' DNA polymerases. Proc Natl Acad Sci U S A. 2010 Nov 8. PMID:21059936 doi:10.1073/pnas.1010436107
- ↑ Nakamura A, Nemoto T, Heinemann IU, Yamashita K, Sonoda T, Komoda K, Tanaka I, Soll D, Yao M. Structural basis of reverse nucleotide polymerization. Proc Natl Acad Sci U S A. 2013 Dec 24;110(52):20970-5. doi:, 10.1073/pnas.1321312111. Epub 2013 Dec 9. PMID:24324136 doi:http://dx.doi.org/10.1073/pnas.1321312111
- ↑ http://www.capture.caltech.edu/