User:Bailey Dove/Sandbox1
From Proteopedia
Contents |
Hepatitis C Virus
The Hepatitis C virus (HCV) is a blood-borne, single stranded RNA virus. [1] Chronic Hepatitis C infection is a leading cause of mortality and morbidity across the globe, affecting almost 170 million people. [2] The genome of HCV encodes a polyprotein precursor of approximately 3,000 amino acids, which are cleaved into 10 proteins by various proteases, one of which is the non-structural protein 3 (NS3) serine protease. [3]
|
Victrelis
Victrelis (boceprevir) is an antiviral Hepatitis C medication approved by the United States Food and Drug Administration in 2011. Victrelis is an inhibitor of the NS3 serine protease.[4] Inhibition of this protease prevents viral replication in Hepatitis C virus infected cells.[5]
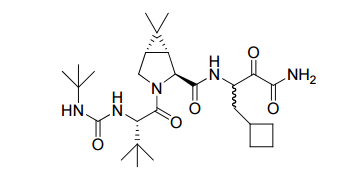
Structure of Victrelis
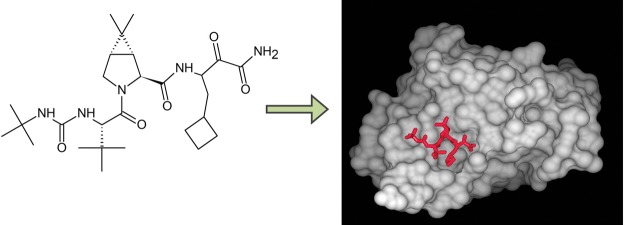
The active ingredient in Victrelis, boceprevir, is an equal mixture of two diastereomers made of entirely organic molecules. The molecular formula is C27H45N5O5, and the molecular weight is 519.8 grams/mole. The structural formula is as follows:[6]
Victrelis covalently, yet reversibly, binds to the NS3 serine protease at the residue.[7] When bound to the NS3 serine protease, Victrelis participates in between the side chains of specific amino acid residues on the NS3 serine protease: Asp168, Arg155, Gly137, Gln41, and Ala156.[8]
Mechanism of Action
Victrelis is a protease inhibitor. A protease is an enzyme that cleaves large polypeptides to create fragments that are small enough to be sequenced for effective replication[9] Victrelis specifically inhibits the HCV NS3/4A protease, which is responsible for cleavage at four different sites along the polyprotein: NS3/NS4A, NS4A/NS4B, NS4B/NS5A, and NS5A/NS5B.[10] The active ingredient in Victrelis, boceprevir, covalently binds to the active site serine () in the NS3 protease. The nucleophilic oxygen in the hydroxyl of the Ser139 group binds to the second carbon from the primary amine group, also known as the beta-carbon.[11] When unbound, Ser139 forms hydrogen interactions with His157 and Asp99 to create a catalytic triad.[12] In previous experiements, when any member of the catalytic triat was replaced by a different residue, cleavage at the NS3/NS4A, NS4A/NS4B, NS4B/NS5A, and NS5A/NS5B locations was inhibited.[13] By inhibiting the protease from cleaving the HCV at the NS3/NS4A location, the polyprotein is unable to be cleaved into smaller, functional proteins that compose replication machinery of HCV, thereby inhibiting viral replication.[14] Mammals also utilize serine proteases to cleave lengthy polypeptides.[15] Victrelis was tested among several different mammalian proteases, including those that are vital for blood clotting, digestion, and antibody neutralization, and it was found to be exclusively selective for inhibition of the NS3 serine protease of HCV only.[16]
References
- ↑ Centers for Disease Control and Prevention. (2015). Viral hepatitis - hepatitis C information. Retrieved from http://www.cdc.gov/hepatitis/hcv/index.htm
- ↑ doi: https://dx.doi.org/10.1177/1756383X11436317
- ↑ Lin C. HCV NS3-4A Serine Protease PMID:21250386
- ↑ Merck & Co., Inc. (n.d.) Highlights of prescribing information. Retrieved from https://www.merck.com/product/usa/pi_circulars/v/victrelis/victrelis_pi.pdf
- ↑ Merck & Co., Inc. (n.d.) Highlights of prescribing information. Retrieved from https://www.merck.com/product/usa/pi_circulars/v/victrelis/victrelis_pi.pdf
- ↑ Merck & Co., Inc. (n.d.) Highlights of prescribing information. Retrieved from https://www.merck.com/product/usa/pi_circulars/v/victrelis/victrelis_pi.pdf
- ↑ Merck & Co., Inc. (n.d.) Highlights of prescribing information. Retrieved from https://www.merck.com/product/usa/pi_circulars/v/victrelis/victrelis_pi.pdf
- ↑ Howe AY, Venkatraman S. The Discovery and Development of Boceprevir: A Novel, First-generation Inhibitor of the Hepatitis C Virus NS3/4A Serine Protease. J Clin Transl Hepatol. 2013 Sep;1(1):22-32. doi: 10.14218/JCTH.2013.002XX. Epub, 2013 Sep 15. PMID:26357603 doi:http://dx.doi.org/10.14218/JCTH.2013.002XX
- ↑ Voet, D., Voet, J., Pratt, C. (2013). Fundamentals of biochemistry: life at the molecular level (4th ed.). Hoboken, New Jersey: John Wiley & Sons, Inc.
- ↑ Lin C. HCV NS3-4A Serine Protease PMID:21250386
- ↑ Merck & Co., Inc. (n.d.) Highlights of prescribing information. Retrieved from https://www.merck.com/product/usa/pi_circulars/v/victrelis/victrelis_pi.pdf
- ↑ Yao N, Reichert P, Taremi SS, Prosise WW, Weber PC. Molecular views of viral polyprotein processing revealed by the crystal structure of the hepatitis C virus bifunctional protease-helicase. Structure. 1999 Nov 15;7(11):1353-63. PMID:10574797
- ↑ Lin C. HCV NS3-4A Serine Protease PMID:21250386
- ↑ Howe AY, Venkatraman S. The Discovery and Development of Boceprevir: A Novel, First-generation Inhibitor of the Hepatitis C Virus NS3/4A Serine Protease. J Clin Transl Hepatol. 2013 Sep;1(1):22-32. doi: 10.14218/JCTH.2013.002XX. Epub, 2013 Sep 15. PMID:26357603 doi:http://dx.doi.org/10.14218/JCTH.2013.002XX
- ↑ Voet, D., Voet, J., Pratt, C. (2013). Fundamentals of biochemistry: life at the molecular level (4th ed.). Hoboken, New Jersey: John Wiley & Sons, Inc.
- ↑ Howe AY, Venkatraman S. The Discovery and Development of Boceprevir: A Novel, First-generation Inhibitor of the Hepatitis C Virus NS3/4A Serine Protease. J Clin Transl Hepatol. 2013 Sep;1(1):22-32. doi: 10.14218/JCTH.2013.002XX. Epub, 2013 Sep 15. PMID:26357603 doi:http://dx.doi.org/10.14218/JCTH.2013.002XX