Sandbox Reserved 563
From Proteopedia
| This Sandbox is Reserved from 05/22/2012, through 07/22/2012 for use in the course "BIOL 414" taught by Greg Buhrman at the North Carolina State University, Raleigh, NC USA. This reservation includes Sandbox Reserved 551 through Sandbox Reserved 590. |
To get started:
More help: Help:Editing |
Hi Linels, Here is your very own Proteopedia Page for pdb code: 3CLN. Your presentation is scheduled for: June 20.
Have Fun!
Greg Buhrman
| |||||||||
| 3cln, resolution 2.20Å () | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | |||||||||
| |||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
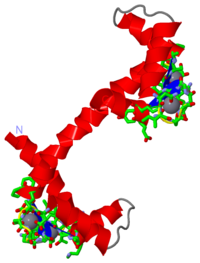
STRUCTURE OF CALMODULIN REFINED AT 2.2 ANGSTROMS RESOLUTION
The crystal structure of mammalian calmodulin has been refined at 2.2 A (1 A = 0.1 nm) resolution using a restrained least-squares method. The final crystallographic R-factor, based on 6685 reflections in the range 2.2 A less than or equal to d less than or equal to 5.0 A with intensities exceeding 2.5 sigma, is 0.175. Bond lengths and bond angles in the molecule have root-mean-square deviations from ideal values of 0.016 A and 1.7 degrees, respectively. The refined model includes residues 5 to 147, four Ca2+ and 69 water molecules per molecule of calmodulin. The electron density for residues 1 to 4 and 148 is poorly defined, and they are not included in the model. The molecule is shaped somewhat like a dumbbell, with an overall length of 65 A; the two lobes are connected by a seven-turn alpha-helix. Prominent secondary structural features include seven alpha-helices, four Ca2+-binding loops, and two short, double-stranded antiparallel beta-sheets between pairs of adjacent Ca2+-binding loops. The four Ca2+-binding domains in calmodulin have a typical EF hand conformation (helix-loop-helix) and are similar to those described in other Ca2+-binding proteins. The X-ray structure determination of calmodulin shows a large hydrophobic cleft in each half of the molecule. These hydrophobic regions probably represent the sites of interaction with many of the pharmacological agents known to bind to calmodulin.
Structure of calmodulin refined at 2.2 A resolution., Babu YS, Bugg CE, Cook WJ, J Mol Biol. 1988 Nov 5;204(1):191-204. PMID:3145979
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
About this Structure
3CLN is a 1 chain structure with sequence from Rattus rattus. This structure supersedes the now removed PDB entry 1cln. The August 2003 RCSB PDB Molecule of the Month feature on Calmodulin by Shuchismita Dutta and David S. Goodsell is 10.2210/rcsb_pdb/mom_2003_8. Full crystallographic information is available from OCA.
Reference
- Babu YS, Bugg CE, Cook WJ. Structure of calmodulin refined at 2.2 A resolution. J Mol Biol. 1988 Nov 5;204(1):191-204. PMID:3145979
Page seeded by OCA on Thu Jan 21 10:12:27 2010