Sandbox Reserved 164
From Proteopedia
bla bla
|
|
Contents |
Superoxide Dismutase 1 (SOD 1)
SOD1 is one of three oxidoreductase enzymes that is responsible for binding copper and zinc ions to highly reactive oxygen free radicals and transforming them into oxygen and hydrogen peroxide. [1] This occurs in a quick two step mechanism:
Cu(2+)SOD + O2− → Cu(+)SOD + O2
Cu(+)SOD + O2− + 2H+ → Cu(2+)SOD + H2O2[2].
This protein is coded for by the SOD 1 gene located on chromosome 21 at position 21q22.1 from base pairs 33,031,934 to 33,041,243. [3]
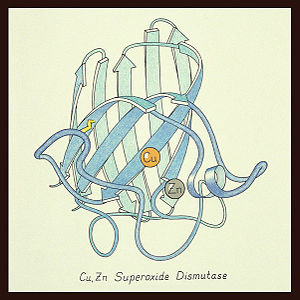
Structure
The SOD 1 protein is a homodimer with an amino acid sequence length of 154. SOD 1 has an 8-stranded "Greek key" beta-barrel shape with the active site located between the barrels. The copper and zinc ligands are made up of six histidine side-chains and one aspartate side-chain with the metal ions connected with by a single histidine chain.[2] This first SOD structure was determined by Irwin Fridovich and Joe McCord in 1973 [1][4] with the SOD 1 "Greek key" structure visualized by Dr. Jane Richardson (see below).[2]
ALS
Mutations to the SOD 1 protein have been linked to the development of familial amyotrophic lateral sclerosis (Lou Gehrig's Disease). [5] These mutations cause a conformational change that leads to motor neuron death through toxic radical build up, promotion of apoptosis, aggregate formation of misfolded proteins, or over stimulation of the cells.[3] In the United States, one of the most common SOD 1 protein mutations is the A4V mutation, where a point mutation causes the alanine at the 4th amino acid position to change to a valine; [6] however, over 100 different mutations have been found in association with the onset of ALS.
References
- ↑ 1.0 1.1 McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049-55. PMID:5389100
- ↑ 2.0 2.1 2.2 Tainer JA, Getzoff ED, Richardson JS, Richardson DC. Structure and mechanism of copper, zinc superoxide dismutase. Nature. 1983 Nov 17-23;306(5940):284-7. PMID:6316150
- ↑ 3.0 3.1 SOD 1. Genetics Home Reference. U.S. National Library of Medicine; 2010
- ↑ McCord JM, Fridovich I. Superoxide dismutase: the first twenty years (1968-1988). Free Radic Biol Med. 1988;5(5-6):363-9. PMID:2855736
- ↑ Al-Chalabi A, Leigh PN (August 2000). "Recent advances in amyotrophic lateral sclerosis". Curr. Opin. Neurol. 13 (4): 397–405. PMID 10970056.
- ↑ Rosen DR, Bowling AC, Patterson D, Usdin TB, Sapp P, Mezey E, McKenna-Yasek D, O'Regan J, Rahmani Z, Ferrante RJ (June 1994). "A frequent ala 4 to val superoxide dismutase-1 mutation is associated with a rapidly progressive familial amyotrophic lateral sclerosis". Hum. Mol. Genet. 3 (6): 981–7. PMID 7951249