NIkkihunter/Sandbox1
From Proteopedia
Ligand Binding N-Terminal of Metabotropic Glutamate Receptors
ABOUT THE MODEL:
This animation represents the Venus flytrap-type-of-action that occurs at the extracellular N-terminal domain, when a binding ligand causes conformational change.
To create this model, two crystallized metabotropic glutamate receptors, in different conformations, from the protein data bank, were homogenized using the Yale Morph Server.
1. 2e4y : hMGluR3 ligand binding domain (mutant) + agonist
2. 2ewk : rMGluR1 ligand binding domain + Glu
ABOUT THE RECEPTORS:
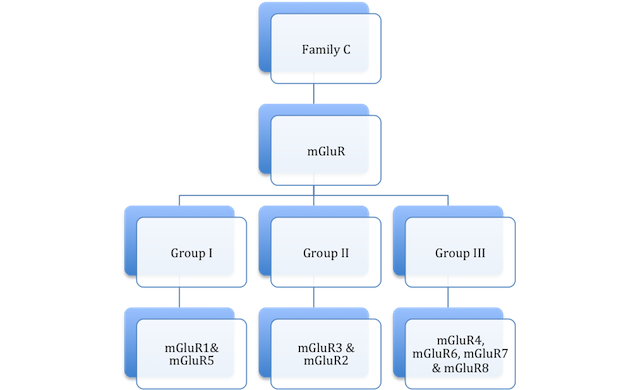
Metabotropic Glutamate Receptors are members of the large class of 7-transmembrane domain receptors, G-Protein Coupled Receptors (GPCR) and the subclass, Family C.
Family C GPCRs have a large extracellular N-terminal (as shown in the animation) that bind to the uninhibited ligand, resulting in the conformational change of the extracellular portion of the receptor. There have been several different ligands identified that have affinity to these sites.
The metabotropic glutamate receptors (mGluRs) play a large role in the alteration of excitatory synaptic transmission in the central nervous system. In short, they affect the activity of other receptors, such as NMDA receptors.
PLACE IN DRUG DISCOVERY:
The mGluR3 receptor has been associated with psychological disorders such as bipolar affective disorder and schizophrenia. The GRM3 gene (the gene encoding for mGluR3) is a likely cause of genetic predisposition to a genetic subtype of bipolar affective disorder making it an area of interest for medicinal research regarding patient specific, customizable, psychiatric medication.
Studies regarding mGluRs suggest they may have some potential in the area of drug research regarding: pain, motor function, memory, autism, neuroimaging and more. Some manipulations suggest it could be an area of interest regarding neuroprotective agents.
Studies with mutant mice have shown mutations in mGluR1 to have possible involvement with certain types of cancer, specifically melanomas.
Resources :
Wellendorph D, Brauner-Osborne H, “Review: Molecular Basis for Amino Acid Sensing by Family C G-Protein-Coupled Receptors.” British Journal of Pharmacology (2009) 156:869-884.
Image from the RCSB PDB (www.pdb.org) of PDB ID of 1EWK (Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, Kumasaka T, Nakanishi S, Jingami H, Morikawa K, “Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor.” (2000) Nature 407: 971-977) created using JMOL
Image from the RCSB PDB (www.pdb.org) of PDB ID of 2E4Y (Muto T, Tsuchiya D, Morikawa K, Jingami H, “Structures of the Extracellular Region of the Group II/ III Metabotropicgltamate Receptors.” (2007) Proc.Natl.Acad.Sci.USA 104:3759-3764) created using JMOL
| |||||||||||