LRRK2
From Proteopedia
Contents |
Parkinson's disease
Parkinson’s disease (PD) is a gradually worsening brain disease accompanied by stiffness, shaking, fatigue, memory difficulties, behavioural changes and problematic walking, coordination, and balance. Movement problems typical for this disease are caused by the death of neurons producing dopamine. Other health issues, such as fatigue, sudden drop in blood pressure, or decreased function of digestive system might be caused by the nerve endings loss of neurons producing norepinephrine. Parkinson´s disorder can be hereditary or occur randomly [1]. Hereditary form of this disease is usually inherited in autosomal dominant pattern [2].
![Top and bottom view of dimeric full-length LRRK2 model with domains indicated in color (Armadillo, white; Ankyrin [ANK], green; LRR, yellow; Roc, blue; COR, violet; kinase, red; WD40, cyan.) Bottom pictures: LRR, yellow; Roc, blue; N-/C-terminal COR subdomains, violet.](/wiki/images/thumb/4/44/FulllengthLRRK2model.jpg/300px-FulllengthLRRK2model.jpg)
LRRK2
This multidomain protein called LRRK2 or dardarin or leucine-rich repeat serine/threonine-protein kinase 2 is a product of LRRK2 gene. Its abbreviation stands for leucine rich repeat kinase 2 and it belongs to ROCO protein family (see also Serine/threonine protein kinase). Although its precise function is unknown, it is proposed that LRRK2 interacts with microtubules, phosphorylates Rab GTPase which marks membranous cargos moving along the microtubules, phosphorylates β-tubulin, moesin, FoxO1, tau, and others [4]. The cargo might be implicated in pathology of Parkinson´s disease [5].
LRRK2 is 2527 amino acids long protein in which we can distinguish N-terminus with scaffold domains such as armadillo, ankyrin, and leucin-rich repeats interaction motifs, and C-terminus with Ras-like GTPase (ROC domain controlling kinase activity of LRRK2), MAPKKK-like kinase domain, COR domain important for dimerization, and scaffold WD40 domain[6]. LRRK2 has both GTPase and kinase activity, mediated by ROC domain and kinase domain, respectively, and the kinase activity is regulated by the ROC domain [7].
COR domain represents a central part in head-to-head orientation when LRRK2 folds into predominant form of globular dimer, where kinase-WD40 modul folds back to domains of N-terminus and thus allows these parts to interact and create autophosphorylation sites [3].
LRRK2 and Parkinson's disease
Mutations in LRRK2 are accompanied by cell loss and α-synuclein aggregates in the form of Lewy bodies and neurite [8]. Mutation in the position R1441C in LRRK2 is considered pathogenic for PD [9]. With other mutations such as R1441G, and Y1699C occurring in ROC and COR domains, it decreases GTPase activity and induces neuronal toxicity [10], [11]. Among LRRK2 mutations that are proven to cause PD, the R1441 is the second most common, and it was found to be substituted by different amino acids [12].
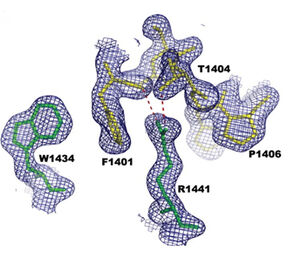
Mechanism of action of R1441C mutation
It was shown that R1441C mutation leads to decrease in the GTPase activity of LRRK2 [14], [15] which results in increase in phosphorylation by this kinase [7]. The R1441C mutation interferes with ROC domain’s GTP-hydrolysing function. Generally, binding of either GDP or GTP stabilizes the protein, the former nucleotide being more effective in this regard. The R1441C mutation affects the stability of the ROC domain as demonstrated by a decrease of melting temperature in comparison to the wild-type protein [16]. This destabilization is less profound with GDP bound than with GTP.
The LRRK2 was proposed to act in the form of a homodimer [17]. The position of R1441C residue was predicted to be at the interface between two monomers in a protein dimeric structure, distal to the active site of the protein – see picture [13]. Mutation in R1441C could therefore destabilize the interaction between monomers and interrupt their enzymatic function [16]. R1441 plays a role in stabilizing the formation of dimer and any mutation at the position R1441 with shorter side chain disrupts both the exquisite hydrogen bonding and stacking interactions provided by the arginine side chain [13]. The interaction between a wild-type ROC domain and ROC mutated at R1441C was shown to be decreased experimentally, simulating the mutation in heterozygous constitution[16]. On top of that, the R1441C mutation has also been shown to improve binding of LRRK2 to neuronal microtubules [18].
3D structures of LRRK2
References
- ↑ Parkinson’s Disease | National Institute on Aging. (accessed Apr. 05, 2021) [1]
- ↑ Parkinson disease: MedlinePlus Genetics (accessed Apr. 05, 2021) [2]
- ↑ 3.0 3.1 Guaitoli G, Raimondi F, Gilsbach BK, Gomez-Llorente Y, Deyaert E, Renzi F, Li X, Schaffner A, Jagtap PK, Boldt K, von Zweydorf F, Gotthardt K, Lorimer DD, Yue Z, Burgin A, Janjic N, Sattler M, Versees W, Ueffing M, Ubarretxena-Belandia I, Kortholt A, Gloeckner CJ. Structural model of the dimeric Parkinson's protein LRRK2 reveals a compact architecture involving distant interdomain contacts. Proc Natl Acad Sci U S A. 2016 Jun 29. pii: 201523708. PMID:27357661 doi:http://dx.doi.org/10.1073/pnas.1523708113
- ↑ Martin I, Kim JW, Lee BD, Kang HC, Xu JC, Jia H, Stankowski J, Kim MS, Zhong J, Kumar M, Andrabi SA, Xiong Y, Dickson DW, Wszolek ZK, Pandey A, Dawson TM, Dawson VL. Ribosomal protein s15 phosphorylation mediates LRRK2 neurodegeneration in Parkinson's disease. Cell. 2014 Apr 10;157(2):472-85. doi: 10.1016/j.cell.2014.01.064. PMID:24725412 doi:http://dx.doi.org/10.1016/j.cell.2014.01.064
- ↑ Abeliovich A, Gitler AD. Defects in trafficking bridge Parkinson's disease pathology and genetics. Nature. 2016 Nov 10;539(7628):207-216. doi: 10.1038/nature20414. PMID:27830778 doi:http://dx.doi.org/10.1038/nature20414
- ↑ Deniston CK, Salogiannis J, Mathea S, Snead DM, Lahiri I, Matyszewski M, Donosa O, Watanabe R, Bohning J, Shiau AK, Knapp S, Villa E, Reck-Peterson SL, Leschziner AE. Structure of LRRK2 in Parkinson's disease and model for microtubule interaction. Nature. 2020 Aug 19. pii: 10.1038/s41586-020-2673-2. doi:, 10.1038/s41586-020-2673-2. PMID:32814344 doi:http://dx.doi.org/10.1038/s41586-020-2673-2
- ↑ 7.0 7.1 Guo L, Gandhi PN, Wang W, Petersen RB, Wilson-Delfosse AL, Chen SG. The Parkinson's disease-associated protein, leucine-rich repeat kinase 2 (LRRK2), is an authentic GTPase that stimulates kinase activity. Exp Cell Res. 2007 Oct 1;313(16):3658-70. Epub 2007 Jul 19. PMID:17706965 doi:http://dx.doi.org/10.1016/j.yexcr.2007.07.007
- ↑ West AB. Achieving neuroprotection with LRRK2 kinase inhibitors in Parkinson disease. Exp Neurol. 2017 Dec;298(Pt B):236-245. doi: 10.1016/j.expneurol.2017.07.019., Epub 2017 Jul 29. PMID:28764903 doi:http://dx.doi.org/10.1016/j.expneurol.2017.07.019
- ↑ Paisan-Ruiz C, Lewis PA, Singleton AB. LRRK2: cause, risk, and mechanism. J Parkinsons Dis. 2013;3(2):85-103. doi: 10.3233/JPD-130192. PMID:23938341 doi:http://dx.doi.org/10.3233/JPD-130192
- ↑ Greggio E, Jain S, Kingsbury A, Bandopadhyay R, Lewis P, Kaganovich A, van der Brug MP, Beilina A, Blackinton J, Thomas KJ, Ahmad R, Miller DW, Kesavapany S, Singleton A, Lees A, Harvey RJ, Harvey K, Cookson MR. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol Dis. 2006 Aug;23(2):329-41. doi: 10.1016/j.nbd.2006.04.001. Epub 2006, Jun 5. PMID:16750377 doi:http://dx.doi.org/10.1016/j.nbd.2006.04.001
- ↑ Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M, Lopez de Munain A, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, de Silva R, Lees A, Marti-Masso JF, Perez-Tur J, Wood NW, Singleton AB. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004 Nov 18;44(4):595-600. PMID:15541308 doi:S0896627304006890
- ↑ Chen ML, Wu RM. LRRK 2 gene mutations in the pathophysiology of the ROCO domain and therapeutic targets for Parkinson's disease: a review. J Biomed Sci. 2018 Jun 14;25(1):52. doi: 10.1186/s12929-018-0454-0. PMID:29903014 doi:http://dx.doi.org/10.1186/s12929-018-0454-0
- ↑ 13.0 13.1 13.2 Deng J, Lewis PA, Greggio E, Sluch E, Beilina A, Cookson MR. Structure of the ROC domain from the Parkinson's disease-associated leucine-rich repeat kinase 2 reveals a dimeric GTPase. Proc Natl Acad Sci U S A. 2008 Feb 5;105(5):1499-504. Epub 2008 Jan 29. PMID:18230735
- ↑ Lewis PA, Greggio E, Beilina A, Jain S, Baker A, Cookson MR. The R1441C mutation of LRRK2 disrupts GTP hydrolysis. Biochem Biophys Res Commun. 2007 Jun 8;357(3):668-71. doi:, 10.1016/j.bbrc.2007.04.006. Epub 2007 Apr 10. PMID:17442267 doi:http://dx.doi.org/10.1016/j.bbrc.2007.04.006
- ↑ Li X, Tan YC, Poulose S, Olanow CW, Huang XY, Yue Z. Leucine-rich repeat kinase 2 (LRRK2)/PARK8 possesses GTPase activity that is altered in familial Parkinson's disease R1441C/G mutants. J Neurochem. 2007 Oct;103(1):238-47. Epub 2007 Jul 10. PMID:17623048 doi:http://dx.doi.org/10.1111/j.1471-4159.2007.04743.x
- ↑ 16.0 16.1 16.2 Li Y, Dunn L, Greggio E, Krumm B, Jackson GS, Cookson MR, Lewis PA, Deng J. The R1441C mutation alters the folding properties of the ROC domain of LRRK2. Biochim Biophys Acta. 2009 Dec;1792(12):1194-7. doi:, 10.1016/j.bbadis.2009.09.010. Epub 2009 Sep 23. PMID:19781641 doi:http://dx.doi.org/10.1016/j.bbadis.2009.09.010
- ↑ Greggio E, Zambrano I, Kaganovich A, Beilina A, Taymans JM, Daniels V, Lewis P, Jain S, Ding J, Syed A, Thomas KJ, Baekelandt V, Cookson MR. The Parkinson disease-associated leucine-rich repeat kinase 2 (LRRK2) is a dimer that undergoes intramolecular autophosphorylation. J Biol Chem. 2008 Jun 13;283(24):16906-14. doi: 10.1074/jbc.M708718200. Epub 2008, Apr 8. PMID:18397888 doi:http://dx.doi.org/10.1074/jbc.M708718200
- ↑ Kett LR, Boassa D, Ho CC, Rideout HJ, Hu J, Terada M, Ellisman M, Dauer WT. LRRK2 Parkinson disease mutations enhance its microtubule association. Hum Mol Genet. 2012 Feb 15;21(4):890-9. doi: 10.1093/hmg/ddr526. Epub 2011 Nov, 11. PMID:22080837 doi:http://dx.doi.org/10.1093/hmg/ddr526
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Adéla Marcalíková, Alexander Berchansky, Jaime Prilusky
