Image:Genes Dev. 2003 Sep 17(18) 2205-32, Figure 1.jpg
From Proteopedia
Size of this preview: 799 × 600 pixels
Full resolution (1058 × 794 pixel, file size: 110 KB, MIME type: image/jpeg)
Summary
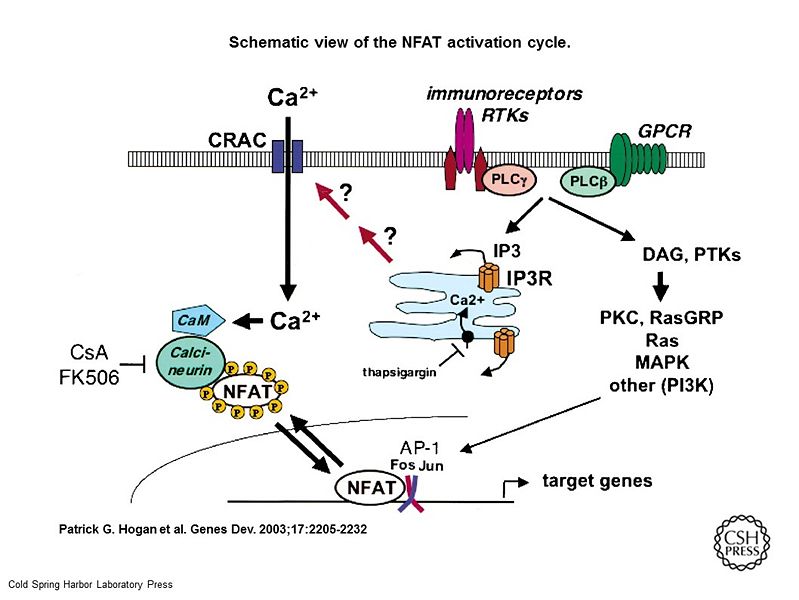
Genes & Dev. 2003. 17: 2205-2232
Schematic view of the NFAT activation cycle. NFAT is activated by cell-surface receptors coupled to Ca2+ mobilization: Immunoreceptors and receptor tyrosine kinases (RTKs) activate phosphatidylinositol-specific phospholipase C (PLC)-γ, while G-protein-coupled receptors (GPCR) activate PLCβ. PLC activation raises intracellular free Ca2+ levels ([Ca2+]I) in several sequential steps: Hydrolysis of phosphatidylinositol (PI)-4,5-bisphosphate (PIP2) by PLC results in generation of inositol-1,4,5-trisphosphate (IP3); IP3 binds to IP3 receptors (IP3R) in the endoplasmic reticulum (blue compartment) and promotes a brief spike of [Ca2+]I increase by depleting ER Ca2+ stores; and store depletion is sensed by an as-yet-uncharacterized signaling mechanism that triggers a sustained process of “store-operated” Ca2+ entry through CRAC channels in the plasma membrane. [Ca2+]I increases result in activation of many calmodulin (CaM)-dependent enzymes, including the phosphatase calcineurin and the CaM-dependent kinases CaMKII and CaMKIV. Calcineurin dephosphorylates multiple phosphoserines on NFAT, leading to its nuclear translocation and activation. Calcineurin activity is inhibited by the immunosuppressive drugs cyclosporin A (CsA) and FK506, which act as complexes with their intracellular immunophilin receptors cyclophilin and FKBP12, respectively. In parallel, hydrolysis of PIP2 by PLC results in production of diacylglycerols (DAG), which activate RasGRP and protein kinase C (PKC). Receptor activation is coupled to activation of protein tyrosine kinases (PTKs), Ras, MAP kinases (MAPK), and PI-3 kinase (PI3K). MAP kinase activation leads to synthesis and activation of Fos and Jun, the components of the heterodimeric transcription factor AP-1, which then binds cooperatively with NFAT to composite NFAT:AP-1 sites found in the regulatory regions of many NFAT target genes. Ca2+ mobilization is terminated by Ca2+-binding proteins and by Ca2+ ATPases in the ER and plasma membranes, which pump Ca2+ back into ER stores and out of the cell, respectively. The ER enzyme is inhibited by thapsigargin, which depletes ER Ca2+ stores and activates CRAC channels in the absence of receptor stimulation. In neurons and vascular smooth muscle cells, NFAT is selectively activated by Ca2+ influx through L-type Ca2+ channels (LTCC; Graef et al. 1999; Stevenson et al. 2001). The underlying mechanism is not understood. Potentially, calcineurin and NFAT could be localized to the vicinity of plasma membrane signaling complexes containing LTCC, NMDA receptors, and scaffold/adapter proteins such as PSD95 and AKAP-79. Alternatively, as suggested for CREB activation, the mechanism could involve selective activation of MAPK pathways via calmodulin bound to LTCC (Dolmetsch et al. 2001).
Licensing
{{subst:Non-commercial from license selector}}
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | User | Dimensions | File size | Comment | |
|---|---|---|---|---|---|
| (current) | 08:11, 14 January 2017 | Camille Zumstein (Talk | contribs) | 1058×794 | 110 KB | Genes & Dev. 2003. 17: 2205-2232 Schematic view of the NFAT activation cycle. NFAT is activated by cell-surface receptors coupled to Ca2+ mobilization: Immunoreceptors and receptor tyrosine kinases (RTKs) activate phosphatidylinositol-specific phospholi |
- Edit this file using an external application
See the setup instructions for more information.
Links
The following pages link to this file:
