Image:4BXC F.trinervia PEPC+G6P crystral structure.png
From Proteopedia
No higher resolution available.
4BXC_F.trinervia_PEPC+G6P_crystral_structure.png (450 × 450 pixel, file size: 823 KB, MIME type: image/png)
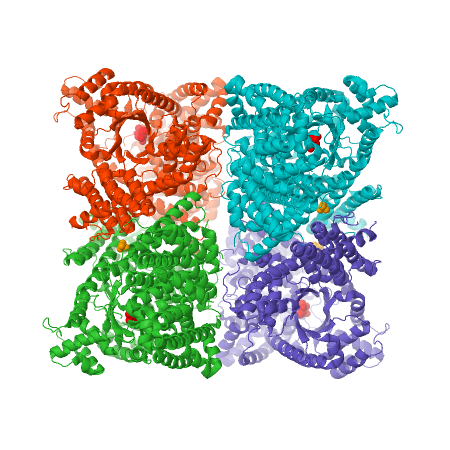
X-ray crystal structure of Flaverina trinervia’s C4 PEPC bound to glucose 6-phosphate (magenta). Two strongly bound dimers (left and right sides of the structure) form the tetrameric quaternary structure. Adapted from Schlieper, D., Förster, K., Paulus, J. K., & Groth, G. (2014). Resolving the Activation Site of Positive Regulators in Plant Phosphoenolpyruvate Carboxylase. Molecular Plant, 7(2), 437–440. doi:10.1093/mp/sst130
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | User | Dimensions | File size | Comment | |
|---|---|---|---|---|---|
| (current) | 19:27, 26 June 2023 | Karsten Theis (Talk | contribs) | 450×450 | 823 KB | |
| 23:36, 25 June 2023 | Lucas Xavier da Cunha (Talk | contribs) | 552×400 | 377 KB | X-ray crystal structure of Flaverina trinervia’s C4 PEPC bound to glucose 6-phosphate (magenta). Two strongly bound dimers (left and right sides of the structure) form the tetrameric quaternary structure. Adapted from Schlieper, D., Förster, K., Paulus | |
| 22:53, 25 June 2023 | Lucas Xavier da Cunha (Talk | contribs) | 1116×809 | 1.54 MB | X-ray crystal structure of Flaverina trinervia’s C4 PEPC bound to glucose 6-phosphate (magenta). Two strongly bound dimers (left and right sides of the structure) form the tetrameric quaternary structure. Adapted from <ref>PMID: 24043710</ref> |
- Edit this file using an external application
See the setup instructions for more information.
Links
The following pages link to this file:
