User:Cameron Evans/Sandbox 1
From Proteopedia
Glutamate Dehydrogenase
Contents |
General Information
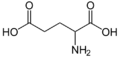
Glutamate Dehydrogenase (GluDH) is a member of the superfamily of amino acid dehydrogenase and functions in the cell to dehydrate α-ketoglutarate to the amino acid glutamate and also to perform the reverse reaction.[1]
GluDH is at the threshold of carbon metabolism (GluDH feeds α-ketoglutarate into the tricarboxylic acid cycle) and nitrogen metabolism (the amine product is utilized by other biosynthetic pathways).[2]. Due to its prominent position on the threshold between catabolic and anabolic pathways, GluDH is ubiquitously expressed in both complex and simple organisms.[3]
In vertebrates and plants, GluDH is preferentially found in the mitochondria, but also in the cytoplasm. In prokaryotes it is found in the cytosol.[4]
Reductive amination of α-ketoglutarate (α-KG) is the process by which the ketone is converted to an amine via an imine intermediate. The reverse reaction, oxidative deamination, is the conversion of the amine functional group to a ketone.
In vertebrates the produced ammonia is usually utilized in the urea cycle and in bacteria the ammonia is assimilated to amino acids and amidotransferases.[5]
Glutamate dehydrogenase shares sequence homology and structural homology to the superfamily of amino acid dehydrogenases, which supports the idea that this superfamily formed by divergent evolution. [1] Because of the homology among all proteins in this superfamily, many dehydrogenases can work on multiple substrates. Nonetheless, GluDH appears to be very specific towards its substrates.
NAD(H) and NADP(H) are cofactors for the reaction and serve to reduce α-KG/ oxidize Glu when they have been oxidized.[2] There are three types of GluDH defined by their preference for cofactor and substrate: (1) NAD(H)-specific GluDH, which mainly catabolizes glutamate and tend to be tetramers; (2) NADP(H)-specific GluDH, which plays a mainly anabolic role; and (3) GluDH with mixed specificity that utilizes both cofactors with similar efficacy. The latter two tend to be hexamers. [1]. Mammalian GluDH are mainly of this third type as they have comprable efficiency for each reaction. Because of the "non-specificity" of mammalian enzymes the protein utilizes allosteric regulation to control metabolic balance. [3]. Prokaryotes do not have allosteric inhibition.[2]
Prokaryote
|
General Structure
Prokaryotic glutamate dehydrogenase (GDH) do not have any common quaternary structure among prokaryotes; however, every prokaryotic structure so far elucidated shows a common overall tertiary structure.
Each monomer (reguardless of quaternary structure) has two domains, each of which have a . Both domains are beta sheets flanked by alpha helices. One domain is a variant of the Rossmann dinucleotide binding fold () and is involved in cofactor binding; while the other domain is involved in the self-assembly of the oligomer (). Furthermore, Domain I contains most of the substrate binding residues (discussed below). A cleft between the domains is responsible for catalytic activity (see section on catalytic activity below). [1]
Specificity
are made up of polar interactions from K89 and S380 and hydrophobic interactions from G90, V377 and A163 to the sidechain.
Glutamate binds within a pocket on the enzyme surface within the catalytic cleft, with its side chain pointed into this pocket.
The key determinant for enzymatic specificity – i.e., what separates GluDH from other dehydrogenases – is the interaction of K89 and S380 with the gamma carboxylate of the substrate. The last three residues that make this interaction are highly conserved among amino acid dehydrogenases. These residues are all within the binding pocket.
Outside of the specificity pocket, the alpha amino of the substrate hydrogen bonds with the main chain carbonyl of D165 and G164, and the alpha carboxylate of the substrate hydrogen bonds to K113 and the side chain of Q110. [1]
The substrate binds deeper within the catalytic cleft than the cofactor. The cofactor binds with its ‘’Re’’ face buried against the enzyme, with its ‘’Si’’ face exposed to the solvent within the catalytic cleft, adjacent to the highly conserved residues responsible for substrate binding. [1] In the highly conserved residues are shown in red and the cofactor is shown in spacefill.
have been found to be critical in shaping this catalytic site – 122, 123, 90, 91, 376
|
Movement
Upon substrate binding, GluDH undergoes an induced change that begins the necessary reaction. This change involves the movement of Domain II some 14 degrees relative to Domain I (which is fixed in the oligomer). This appears to "close" the cleft in which the substrate and the cofactor are bound. To fully terminate the reaction, the movement of the domains in the opposite direction (i.e., to the "open conformation") is required and the products are released.
On the right is an approximation of this movement from available crystal structures. Domain II is highlighted in Blue and Domain I in Purple.()()
Eukaryote
|
General Structure
Overall, mammalian GluDH and prokaryotic GluDH do not differ much in general structure. Furthermore, Bovine GluDH (boGluDH; the most widely studied mammalian GluDH) shares 27% sequence similarity to C. symbiosum GluDH (csGDH) discussed above.[3]
Like most prokaryotic GluDH, Mammalian GluDH has been found to hexamerize as a dimer of .
Furthermore,each of mammalian GluDH, like prokaryotic GluDH, is composed of two domains: Domain I, which is responsible for the assembly of the hexamer; and Domain II, which is responsible for dinucleotide binding (both true statements for prokaryotic GluDH). Furthermore, each domain is similar to the domains within csGluDH as .
Unlike csGluDH, the boGluDH monomer has 48 residue that assists in the trimerization process. . These antennae appear to undergo conformational changes as the "mouth" of GluDH opens and closes.
This 48 residue insertion (397-444), which does not exist in the sequences of prokaryotic GluDH, is thought to be significant for the allosteric interactions that distinguish mammalian GluDH from prokaryotic GluDH (see specific interactions below).[3]
Specificity
GluDH makes non-coavalent and specific contacts with its substrates, cofactors and allosteric inhibitors.
Glutamate and α-KG both bind via hydrogen bonding within the catalytic cleft between the two distinct clamping domains. Both make contact via hydrogen binding with K126, K90, S381, R211, and N349. However, α-KG binding is thought to be stabilized also by N374 (through a water molecule) and K114 and Glu by K114 in a different crystal structure.
Four basic residues are neutralized as the catalytic cleft closes and either reaction begins: K114, 126, 90 and 113. These make specific contacts with the alpha and gamma carboxyl groups of the substrate.
NADH, as it binds within the to the catalytic cleft, makes specific hydrogen bonds with D168, S170, E275, S276, N349, A326 and S327 in all crystal structures. Q250 contacts both NADPH and NADH, but not NAD+. Instead Q330 makes a similar contact to NAD+ in that region.[2]
Allosteric Interactions
Also unlike prokarytoic GluDH, Mammalian GluDH is allosterically controlled by GTP (-), ATP (-), GDP(+) and ADP(+), among other agents that are not likely used in a cell's natural process. It is thought that there is one large region responsible for the binding of allosteric inhibitors between the catalytic core and the helix on which the two domains pivot (residues 445-470). Allosteric inhibitors act as a "doorstop," locking GluDH in a closed position and increase product affinity. On the other hand, positive allosteric interactions are thought to force GluDH into the open conformation, decreasing product affinity.[2]
Nucleotides
The between the active site of the monomer and the pivot helix . It is thought that GTP causes negative regulation of GluDH by increasing the enzyme's affinity for the product to the extent that the release of the product is the rate limiting step of the overall reaction.
When the enzyme is highly saturated, the enzyme has been found to form an "abortive complex" that is the cofactor and the reagent locked in a non-catalytic conformation. Upon the binding of a positive regulator, like GDP or ADP, the protein is thought to be forced into the open conformation, driving the reaction is allowed to completion. ADP has also been shown to decrease the affinity of the enzyme to its products.
via hydrogen bonding. Most of the contacts are with the triphosphate moeity - the sidechains of H209, H450, Y262, R217, R265, R261 - however, the sidechains of K281 and E292 make specific contacts with the adenosine ring (to the carbonyl and the N1 imino, respectively) and S213 makes contact with the 2' hydroxyl on the sugar. The guanidinium of R261 is thought to stack against the purine ring. ADP is thought to bind in a similar way as GTP and ADP have been found to bind antagonistically in competitive studies. [2]
Dinucleotides
The NAD(P)(H) coenzyme is has also been shown to - that is, between the pivot helix and the active site. Furthermore, this binding site appears to bind NAD(H) with ten times the affinity as NADP(H). As might be expected, the binding of the reduced coenzyme inhibits oxidative deamination, and the binding of the oxidized form promotes deamination.
During reductive amination, NADPH is an inhibitor at pH 8, but not at pH7. Furthermore, NADPH binding is greatly enhanced in the presence of glutamate, where it inhibits the enzyme; however, the enhanced binding observed in the presence of ketoglutarate is not coupled with inhibition.
The following residues have been shown to be important in the binding of the secondary cofactor: Y190, K143, K428, K425 and Y412.[3]
Catalytic Mechanism
The catalytic mechanism for all crystal complexes of GluDH have not been elucidated. Peterson and Smith (1999) have proposed a model for cleft closing of bovine GluDH in four steps.
(1) In the hydrophobic cleft, a lysine (126) has an unusually low pKa. This lysine loses a proton to the solvent and initiates the closing of the catalytic cleft.(2) A water hydrogen bonds to the amine of the lysine and (3)expels the bulk solvent from the catalytic cleft. (4) Subsequently, the alpha hydrogen of the substrate is brought into close proximity with the oxidized substrate, allowing hydride transfer.
In prokaryotes, this model has been found to be slightly different as the deprotonation of the basic residue comes after the fourth step. [3]
In the apo form of csGluDH, K113 (domain I) is hydrogen bound to N373 (domain II) and stabilizes the open structure. On substrate binding, K113 moves to hydrogen bond to the alpha carbonyl of the substrate while maintaining contact with N373. This causes the conformational change which closes the cleft. Based on the crystal structures of csGluDH and previous binding studies with boGluDH, Stillman and Baker, ‘’et al’’ (1993) have proposed the following catalytic mechanism.
After substrate binding and monomer closing, (1) the alpha amino of the glutamate is deprotonated by E165, and (2) hydride transfer to the ‘’Si’’ face of the coenzyme. (3) The change in substrate geometry is sensed by K133and the closed conformation is thought to be brought even closer together to facilitate hydride transfer. (4) Water attacks the iminoketoglutarate intermediate (to become the carbonyl oxygen) and (5) the protons gained by K125 and D165 in catalysis are lost and the monomer returns to the open conformation. (The Principle of Microreversability dictates that the mechanism for reductive amination can be easily described in a backwards fashion). [1]
The details of how ammonia fits into this mechanism remain unknown.[5]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Stillman TJ, Baker PJ, Britton KL, Rice DW. Conformational flexibility in glutamate dehydrogenase. Role of water in substrate recognition and catalysis. J Mol Biol. 1993 Dec 20;234(4):1131-9. PMID:8263917 doi:http://dx.doi.org/10.1006/jmbi.1993.1665
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Smith TJ, Peterson PE, Schmidt T, Fang J, Stanley CA. Structures of bovine glutamate dehydrogenase complexes elucidate the mechanism of purine regulation. J Mol Biol. 2001 Mar 23;307(2):707-20. PMID:11254391 doi:10.1006/jmbi.2001.4499
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Peterson PE, Smith TJ. The structure of bovine glutamate dehydrogenase provides insights into the mechanism of allostery. Structure. 1999 Jul 15;7(7):769-82. PMID:10425679
- ↑ EC 1.4.1.2 Brenda 2010
- ↑ 5.0 5.1 Lightfoot DA, Baron AJ, Wootton JC (1988). "Expression of the Escherichia coli glutamate dehydrogenase gene in the cyanobacterium Synechococcus PCC6301 causes ammonium tolerance". Plant Molecular Biology 11 (3): 335-344. doi 10.1007/BF00027390