Tomato aspermy virus protein 2b Suppression of RNA Silencing
From Proteopedia
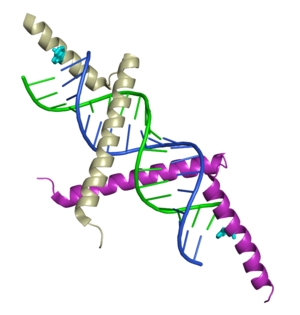
3D structure of Tomato aspermy virus protein 2b complexed to siRNA
Contents |
Background
RNA silencing is a gene inactivation system in many eukaryotes that relies on tiny RNAs as the targeting molecules. One function of RNA silencing, which is also called post-transcriptional gene silencing (PTGS) or RNA interference (RNAi), is to act in surveillance against molecular parasites, such as viruses. Double-stranded RNA triggers the RNA silencing pathway and most plant viruses use a double-stranded RNA to replicate their genome. Various plant viruses have developed evasion techniques to circumvent this surveillance system. In one such evasion strategy, Tomato aspermy virus protein 2b suppresses a plant's anti-viral RNA-induced silencing response[1].
siRNAs are generally characterized by their short length (21–26 nt), 2 nt, 3′ overhanging ends, and 5′ phosphate groups. The most efficient silencing is obtained with siRNA duplexes composed of 21-nt sense and 21-nt antisense strands, paired in a manner to have a 2-nt 3' overhang (see the Tuschl lab's guide for designing siRNAs)[2][3].
A structural study [4] has revealed how Tomato aspermy virus protein 2b directly binds the double-stranded siRNA.
Results
| |||||||
| TAV2b and siRNA 2zi0, resolution 2.82Å () | |||||||
|---|---|---|---|---|---|---|---|
| Resources: | FirstGlance, OCA, PDBsum, RCSB | ||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||
The X-ray crystal structure of includes both protein and RNA in the complex.
The .
The synthetic RNA substrate mimics a double-stranded siRNAs that occur in the double-strand RNA-induced RNAi silencing pathway.
The duplex region is 19 nts and a normal biological siRNA also has additional 2-nt 3' overhangs.
Here is a schematic illustration of the two strands of the silencing RNA (siRNA) mimic in this structure:
5'-pAGACAGCAUUAUGCUGUCU-OH-3'
||||||||| |||||||||
3'-OH-UCUGUCGUAUUACGACAGAp-5'
The TAV2b protein binds the siRNA as
.
Individual monomers of the TAV2b dimer are colored tan and purple.
Each is made of two two alpha helices connected by a short loop.
The TAV2b dimer forms .
.
The helices of the TAV2b dimer sit deep the major groove of the duplex region of the double stranded siRNA. This is in contrast to other means used to recognize siRNAs, for example, see 1rpu or 1r9f and 2az0. The major groove of A-form RNA is deep and narrow, unlike in DNA where the major groove is wide so that the edges of bases are more accessible for base-specific contacts. In fact, although there is no significant interaction with the 2'-hydroxyls of the siRNA, TAV2b distinguishes between dsRNA and dsDNA by measuring the width of the major groove with its helical backbone. [Note: this view generates a substantial surface which may take half a minute to calculate.]
.
The TAV2b dimer seems to accurately measure two helical turns of dsRNA by simultaneously recognizing two adjacent major grooves by its unique pair of hook-like structures. [Note: this view generates a substantial surface which may take half a minute to calculate.]
.
Where the protein backbone of the N-terminal alpha-helix fits into the major grooves, a number of positively charged, conserved residues (R26, H29, N32, R33, R36) form hydrogen bonds and electrostatic interactions with the phosphate backbone of both strands of the RNA. Another, more C-terminal patch of conserved amino acids (K29, S40, Pro41, S42, E43) forms hydrogen bonds with the backbone of the distal portions of the major groove.
. Somewhat reminiscent of p19's interaction with siRNA, the TAV2b dimer has two tryptophan residues (W50) that stack over the 5' terminal base of each strand of the siRNA. This tryptophan is only relatively conserved and in vitro binding studies where it is mutated little loss of binding affinity.
.
One set of crystallographic contacts in the structure forms a TAV2b tetramer; the investigators reported it might exist in solution as well, and therefore could be biologically relevant to suppression of RNA silencing by the virus.
Conclusions
The X-ray crystal structure shows TAV2b recognizes siRNA in a manner very distinctive from that seen for p19 and viral protein B2. TAV2b recognizes siRNA by fitting alpha-helices into the RNA major groove and wrapping around both faces of the RNA to recognize the major groove backbone. p19 and viral protein B2 and all other known double-stranded RNA-binding proteins only bind one face to recognize the major or minor groove. Alpha-helix docking into the major groove is more familiar as a strategy for high-specificity binding of DNA (see for example, 6cro, 2qhb, 1puf, 1gd2, 1nwq 1f4k, 3ere), and even of RNA (see 1etg[5]; albeit the structure is uniquely distorted), with non-sequence-specific binding involving helix docking into the major groove, as seen for TAV2b complexed to siRNA, being less characterized. Thus, TAV2b uses a unusual mechanism where its hook like dimers interact with the backbone of two helical turns of RNA to recognize the siRNA duplex in a sequence-independent and length-preferential manner. The structural and in vivo characterization suggests TAV2b blockes virus invasion by binding siRNA duplexes, preventing them from loading into the RNA-induced silencing complex. However, 2b proteins may not be limited to RNA binding and may in fact bind to an inhibit the protein Argonaute, which is also critical for RNA silencing [6], and thus could help block host RNA silencing in multiple ways to aid viral infection.
About this Structure
2zi0 is a Protein complex structure of sequences from Tomato aspermy virus. Full crystallographic information is available from OCA. The molecular weight of TAV2b protein chain designated 'a' seen in the solved structure is 7.3 kDa (60 residues visible). The molecular weight of the TAV2b protein chain designated 'b' seen in the solved structure is 6.8 kDa (55 residues visible). Total size of the TAV2b-RNA complex seen in the structure is 31.5 kDa. Biological TAV2b is 95 amino acids and recombinant TAV2b used to generate the crystals was residues 1-69.
Reference for the Structure
Structural basis for RNA-silencing suppression by Tomato aspermy virus protein 2b., Chen HY, Yang J, Lin C, Yuan YA, EMBO Rep. 2008 Jul 4;. PMID:18600235
Related Structures and Topics
- Suppression of RNA Silencing by Viruses
- Plant Viral Protein p19 Suppression of RNA Silencing
- Flock house virus B2 protein Suppression of RNA Silencing
- 2zi0 Tomato aspermy virus protein 2b bound to siRNA
- 1rpu Carnation italian ringspot virus p19 bound to siRNA
- 1r9f Tomato bushy stunt virus p19 bound to siRNA
- 2az0 Flock house virus B2 protein bound to double-stranded RNA (dsRNA)
- 2b9z Flock house virus B2 protein solution structure
- RNA interference
Notes and Literature References
- ↑ Suppression of post-transcriptional gene silencing by a plant viral protein localized in the nucleus., Lucy AP, Guo HS, Li WX, Ding SW, EMBO J. 2000 Apr 3;19(7):1672-80. PMID:10747034
- ↑ Functional anatomy of siRNAs for mediating efficient RNAi in Drosophila melanogaster embryo lysate., Elbashir SM, Martinez J, Patkaniowska A, Lendeckel W, Tuschl T, EMBO J. 2001 Dec 3;20(23):6877-88. PMID:11726523
- ↑ Zhang X, Du P, Lu L, Xiao Q, Wang W, Cao X, Ren B, Wei C, Li Y. Contrasting effects of HC-Pro and 2b viral suppressors from Sugarcane mosaic virus and Tomato aspermy cucumovirus on the accumulation of siRNAs. Virology. 2008 May 10;374(2):351-60. Epub 2008 Feb 15. PMID:18280529 doi:http://dx.doi.org/10.1016/j.virol.2007.12.045
- ↑ Structural basis for RNA-silencing suppression by Tomato aspermy virus protein 2b., Chen HY, Yang J, Lin C, Yuan YA, EMBO Rep. 2008 Aug;9(8):754-60. Epub 2008 Jul 4. PMID:18600235
- ↑ Battiste JL, Mao H, Rao NS, Tan R, Muhandiram DR, Kay LE, Frankel AD, Williamson JR. Alpha helix-RNA major groove recognition in an HIV-1 rev peptide-RRE RNA complex. Science. 1996 Sep 13;273(5281):1547-51. PMID:8703216
- ↑ Zhang X, Yuan YR, Pei Y, Lin SS, Tuschl T, Patel DJ, Chua NH. Cucumber mosaic virus-encoded 2b suppressor inhibits Arabidopsis Argonaute1 cleavage activity to counter plant defense. Genes Dev. 2006 Dec 1;20(23):3255-68. PMID:17158744 doi:http://dx.doi.org/20/23/3255
Additional Literature and Resources
- Tour of p19 bound to an siRNA by Wayne Decatur, in an exploration-friendly interface that is adapted from Eric Martz's FirstGlance in Jmol
- Structural basis for RNA-silencing suppression by Tomato aspermy virus protein 2b., Chen HY, Yang J, Lin C, Yuan YA, EMBO Rep. 2008 Aug;9(8):754-60. Epub 2008 Jul 4.PMID:18600235
- The structure of the flock house virus B2 protein, a viral suppressor of RNA interference, shows a novel mode of double-stranded RNA recognition., Lingel A, Simon B, Izaurralde E, Sattler M. EMBO Rep. 2005 Dec;6(12):1149-55. PMID:16270100
- Dual modes of RNA-silencing suppression by Flock House virus protein B2. Chao JA, Lee JH, Chapados BR, Debler EW, Schneemann A, Williamson JR, Nat Struct Mol Biol. 2005 Nov;12(11):952-7. PMID:16228003
- Recognition of small interfering RNA by a viral suppressor of RNA silencing., Ye K, Malinina L, Patel D J, Nature 2003 426(6968):874-878. Epub 2003 Dec 3. PMID:14661029
- Sizing up small RNAs., Jabri E, Nature Structural & Molecular Biology 2004 11: 112. PMID:14749769
- Crystal structure of p19--a universal suppressor of RNA silencing., Baulcombe DC, Molnar A, Trends Biochem Sci. 2004 29(6):279-281. PMID:15276178
- Plant viral suppressors of RNA silencing., Roth BM, Pruss GJ, Vance VB, Virus Res. 2004 Jun 1;102(1):97-108. PMID:15068885
- Novel modes of protein-RNA recognition in the RNAi pathway. Lingel A, Sattler M, Curr Opin Struct Biol. 2005. 15(1):107-115. PMID:15718141
- Nature Reviews RNAi collection
- The Tombusvirus-encoded P19: from irrelevance to elegance.,Scholthof HB, Nature Reviews Microbiology. 2006 May;4(5):405-11. PMID:16518419
- Featured on the May 2009 cover of the journal RNA
Categories: RNA Silencing | Post-transcriptional gene silencing (PTGS) | Virus | Plant Virus | Double-stranded RNA (dsRNA) | SiRNA | Small Interfering RNA | Tomato aspermy virus | Single protein | Burgyan, J. | Chen, H Y. | Yuan, Y A. | Coiled coil | Gene regulation/rna complex | Protein-rna complex | Protein complex | Rna double helix | A-form helix | Rna length recognition | Rnai | Nucleus | Rnai suppression | Suppressor of rna silencing | Topic Page