Sonia Ferveur/Sandbox
From Proteopedia
Contents |
Ovalbumin
|
Ovalbumin (1OVA) is the main protein of the avian egg white. This protein represent approximatively 60% of the total protein. Its exact function is still unknown but it is thought to be a storage protein for the developing foetus. Storage proteins are a reserve of ion and amino acid which can be used by organisms.
Ovalbumin has sequence similarities and three dimensional homology with the serpin superfamily. Serpin are serine protease inhibitor but some of them have also non inhibitory roles, such as ovalbumin which belongs to the subgroup known as ov-serpins (ovalbumin-related serpins) or class B serpins.
In the human, ov-serpins include 13 proteins involved in the regulation of inflammation, apoptosis, angiogenesis, and embryogenesis. It suggests that “newer” serpins, such as ovalbumin, have contributed to vertebrate adaptation. [1]
Structure
The first three dimensional structure of the chicken ovalbumin has been established with the molecular replacement method using the structure of the plakalbumin (a cleaved form of ovalbumin). The final structure has been resolved by crystallography and was the first crystal structure of a member of the serpin family in an uncleaved form. [2]
Ovalbumin is a 42.8kDa protein consisting of 386 residus. Ovalbumin can form a .
It has a characteristic serpin domain consisting of 8-9 alpha helix and 4 beta sheets. Ovalbumin lacks a conformational change in the , which confers the characteristics of a protease inhibitor to the serpin family [3]. This change involves the partial insertion of the reactive center loop (in yellow Fig 1A) into the located in the center of the protein (shown in yellow Fig 1B). [4]
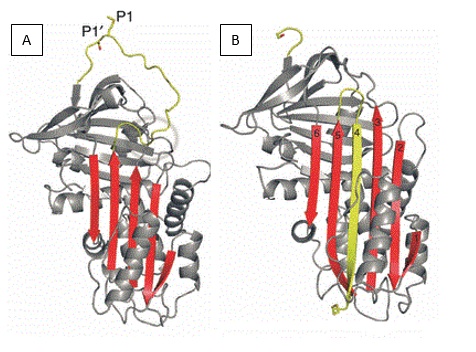
Fig1: Structures of native and stable inhibitory serpins
As for ovalbumin, the insertion of the reactive center loop in the β-sheet region is impossible because of a substitution of a threonine in an arginine at a critical point. Therefore ovalbumin is a non-inhibitor serpin.
Ovalbumin tends to change into a more stable protein: S-ovalbumin.[5]
Function
Ovalbumin takes a key role during the eggshell formation where it could serve as an effective stabilization agent for some precursors and could prevent the mineralization of the eggshell at the early state of development. [6]
Properties
Ovalbumin can coagulate when heated: in solution at a concentration higher than 0.1% heated, a coagulum is formed [7].
Its isoelectric point has been established at 4.7 (pI=4.7). [2]
Disease
Ovalbumin is one of the major allergens causing some children under 4 years old to be highly sensitized to egg white. Ovalbumin sensitization is related to atopic dermatitis and children with ovalbumin sensitization are more likely to have asthma with atopic dermatitis, atopic dermatitis with allergic rhinitis, or atopic dermatitis, but are less likely to have asthma with allergic rhinitis. Atopic or allergic diseases causes a decrease of the titer of ovalbumin-IgG4 antibodies but an increase of ovalbumin-IgE antibodies. [8]
Biotechnological use
Ovalbumin is used to induce asthma in rat, mouse and guinea pig to assess the in vivo efficacy of anti-asthma drugs. [9]
Ovalbumin is also used by industries and for the research about vaccination. Indeed, this glycoprotein is enough large and complex to be immunogenic. Consequently, it is widely used as an antigen for immunization research. [10]
References
- ↑ Charaf Benarafa and Eileen Remold-O'Donnell (2005) "The ovalbumin serpins revisited: Perspective from the chicken genome of clade B serpin evolution in vertebrates", Proc Natl Acad Sci USA doi:10.1073/pnas.0502934102
- ↑ Stein, P.E., Leslie, A.G., Finch, J.T., Carrell, R.W. (1991) "Crystal structure of uncleaved ovalbumin at 1.95 A resolution.", J Mol Biol. 1942038
- ↑ James A. Huntington & Penelope E. Stein (2001), "Structure and properties of ovalbumin", Journal of Chromatography B: Biomedical Sciences and Applications Vo756, p189–198 S0378-4347(01)00108-6
- ↑ J. A. HUNTINGTON (2011), "Serpin structure, function and dysfunction", Jo. of Thrombosis and haemostasis, Vo9doi:10.1111/j.1538-7836.2011.04360.x
- ↑ J. A. Huntington, P. A. Patston, and P. G. Gettins (1995) "S-ovalbumin, an ovalbumin conformer with properties analogous to those of loop-inserted serpins", Protein Science doi:10.1002/pro.5560040403
- ↑ Stephan E. Wolf and al. (2011), "Strong stabilization of liquid amorphous calcium carbonate by ovalbumin: gaining insight into the mechanism of ‘polymer-induced liquid precursor’ processes", J. Am. Chem. Soc., no133 doi:10.1021/ja202622g
- ↑ Donald D. Bills and al. (2014) Biotechnology and Food Safety: Proceedings of the Second International Symposium, UM-USDA-DUPONT, 381 p. GoogleBook
- ↑ Yang-Te Lin and al. (2013)"Correlation of ovalbumin of egg white components with allergic diseases in children" Jo of microbiology, immunology and infection 10.1016/j.jmii.2014.01.002
- ↑ Charles River, OVA
- ↑ Invivogen, OVA antigens
