Molecular Playground/DnaK
From Proteopedia
|
One of the CBI Molecules being studied in the University of Massachusetts Amherst Chemistry-Biology Interface Program at UMass Amherst and on display at the Molecular Playground.
Molecular Playground banner: DnaK, a central hub in maintaining proteostasis in E. coli
The E. coli Hsp70, DnaK, is a protein chaperone whose function is to bind exposed hydrohpobic residues of unfolded proteins. This binding event prevents aggregation and rescues the nascent chain from kinetic traps along the folding pathway. Hsp70 protein chaperones switch between an , low-substrate affinity form and an , high substrate affinity form during their allosteric cycle.1 Hsp70 protein chaperones are ubiquitously found in almost all known organisms and cell types and represent a potential target for anti-cancer and neurodegenerative therapies.2
Contents |
Structure
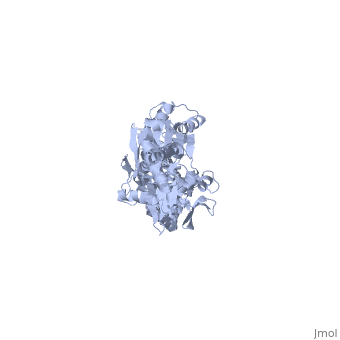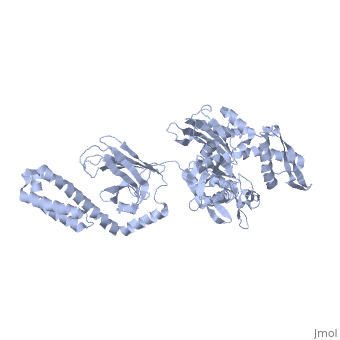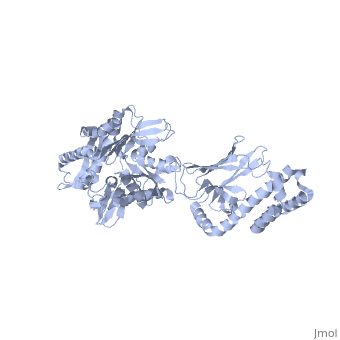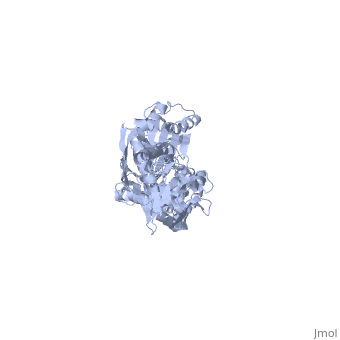
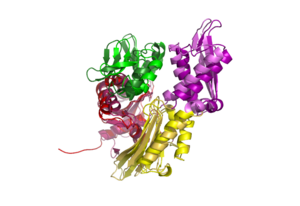
DnaK is a 638 residue protein of approximately 70 kDa. The protein can be thought to be made up of two domains, the N-terminal nucleotide-binding domain () (residues 1-388), the C-terminal substrate-binding domain () (residues 393-638), which are divided by the (residues 389-392, shown in violet). The NBD is further divided into four subdomains: (residues 1-37, 112-184, 363-383, shown in red), (residues 38-111, shown in green), (residues 185-227, 310-362, shown in gold), and (residues 228-309, shown in purple). The SBD of DnaK consists of a (residues 393-507), an (residues 508-605), and a disordered C-terminal tail (not shown due to lack of available crystal structure).3
Function
DnaK binds stretches (7-8 residues in length) of exposed hydrophobic residues of its client proteins, in the beta-basket of the SBD, in order to prevent their aggregation.4 Upon ATP binding, the NBD subdomains rotate relative to each other and induce a conformational change in the NBD (compare the NBD to the form in Figure 1.5Drug Target
Since Hsp70s are critically positioned in the proteostasis systems of many organisms, including humans, they represent a tempting target for anti-cancer and neurodegenerative disease therapies. Recent efforts to develop a competitive inhibitor for Hsp70s, using DnaK as a model system, have met with only marginal success.11 Recently, interactions between Hsp70s and its co-chaperones have been targeted for drug development, but with no leads currently in clinical trials.12
3D structures of Hsp70
See Also
The Wikipedia page on Hsp70 is useful for a general background.14
This informational video demonstrates the successful folding of a protein in E. coli
The Gierasch lab, in collaboration with the Powers lab at Scripps, has developed a computational model of the proteostasis network in E. coli called FoldEco.15 A web version of FoldEco can be found here
References
1. Bertelsen EB. et al. Proc Natl Acad Sci USA 2009
2. Broer L. et al. J Alzherimers Dis 2011
3. Zuiderweg ER. et al. Top Curr Chem 2013
4. Wegele H. et al. Rev Physiol Biochem Pharmacol 2004
5. Zhuravleva A. et al. Proc Natl Acad Sci USA 2011
6. Swain JF. et al. Mol Cell 2007
7. Kityk, R. et al. Mol Cell 2012
8. Mayer MP. Trends Biochem Sci 2013
9. Sharma SK. et al. Nat Chem Bio 2010
10. Zhuravleva A. et al. Cell 2012
11. Li X. et al. ACS Med Chem Lett 2013