Human RecQ-Like protein 1
From Proteopedia
Contents |
RECQL (RECQ1): Human RecQ-Like protein 1
| |||||||||
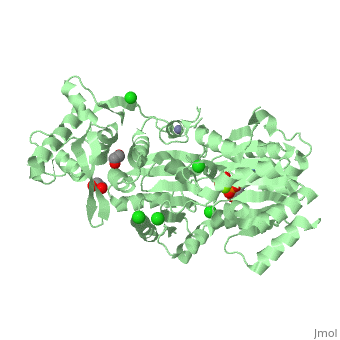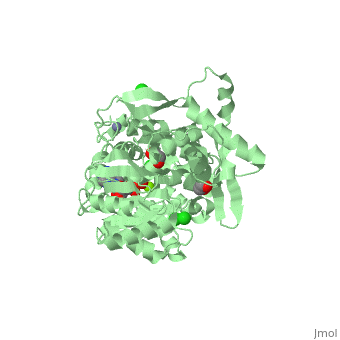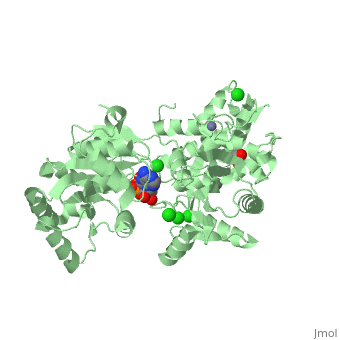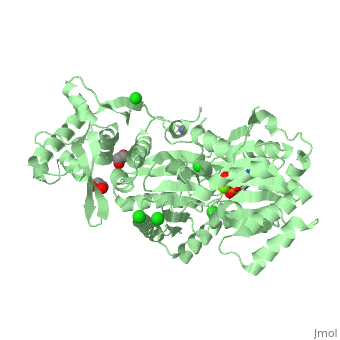
| Human RecQ-like protein 1 complex with ADP, ethane diol, ethylene glycol, Mg, Zn, Cl, 2v1x | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligands: | , , , , | ||||||||
| |||||||||
| |||||||||
| Resources: | FirstGlance, OCA, RCSB, PDBsum | ||||||||
| Coordinates: | save as pdb, mmCIF, xml | ||||||||
The RecQ family of DNA helicases are conserved in organisms from bacteria to man (1,2). A single RecQ protein is produced in E.coli and yeast, whereas the human genome has five distinct family members: RECQL (RECQ1), BLM, WRN, RECQL4 and RECQL5.
Mutations in the BLM, WRN and RECQL4 genes cause genetic disorders that share a high incidence of cancer and chromosomal instability, as well as gene specific defects such as premature aging and developmental defects. Although not directly linked to human disease, the RECQL protein has been shown in vitro to be important in Holliday branch migration and in the regulation of recombination and chromosome stability.
The RecQ proteins share a core activity, ATP-dependent unwinding of DNA duplexes in the 3’-5’ direction. Forked DNA structures are preferred substrates for most family members, while other DNA structures such as G-rich tetrastrands, Holliday junctions and tailed duplexes, are differentially utilized by the different enzymes. All RecQ helicases share the widely conserved helicase/ATPase motifs as well as a more C-terminal segment that is characteristic of the RecQ family (RecQ-Ct). A Helicase and RNase D C-terminal (HRDC) domain, involved in DNA binding, is present in some RecQ helicases (E. coli RecQ, S. cerevisiae SGS1) but absent in RECQL, RECQL4 and RECQL5. Some RecQ proteins have extensive non-homologous regions involved in protein interactions and additional enzymatic activities.
RECQL can exist as dimers or assemble into larger oligomers. Biochemical studies have assigned different activities to the monomeric and oligomeric forms. (3,4). A trimmed version of human RECQL, lacking 48 N-terminal and 25-33 C-terminal amino acids, was expressed in bacteria and crystallized. The protein exists exclusively as a dimer in solution and exhibits the helicase activity associated with the dimeric form of full-length RECQL, but not activities of the oligomeric protein.
The crystal structure of RECQL in complex with Mg-ADP shares the domain organization of the bacterial homologue RecQ. However, the relative position of the domains is altered: most prominently, the C-terminal winged-helix domain is completely re-oriented relative to the conserved ATPase domain. These differences may indicate that a major conformational change occurs during the active cycle of RECQ helicases.
Additional Resources
For additional information, see: DNA Replication, Repair, and Recombination
3D structures of helicase
References
- Hickson, I.D. (2003) Nat. Rev. Cancer 3:169-78.
- Bennett, R.J. and Keck, J.L. (2004) Crit. Rev. Biochem. Mol. Biol. 39:79-97.
- Sharma. S. et al. (2005) J. Biol. Chem. 280:28072-28084.
- Muzzolini, L et al. (2007) PLoS Biol. 5:e20.
- Bernstein, D.A., Zittel, M.C. and Keck, J.L (2003) EMBO J. 22:4910-4921.