1s9v
From Proteopedia
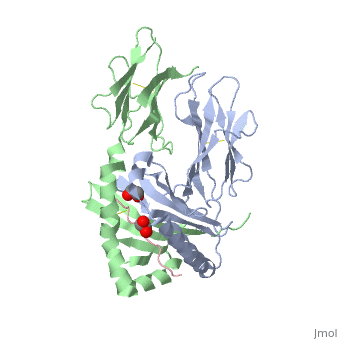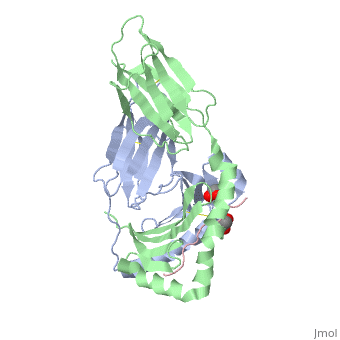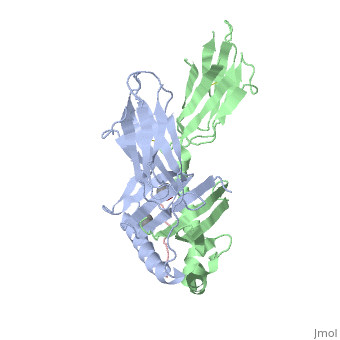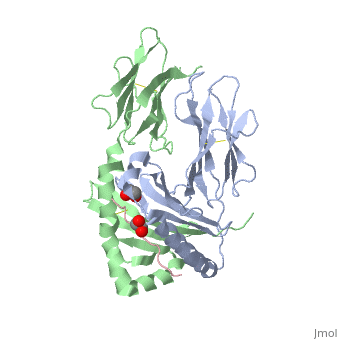
Crystal structure of HLA-DQ2 complexed with deamidated gliadin peptide
Structural highlights
Function[DQA1_HUMAN] Binds peptides derived from antigens that access the endocytic route of antigen presenting cells (APC) and presents them on the cell surface for recognition by the CD4 T-cells. The peptide binding cleft accommodates peptides of 10-30 residues. The peptides presented by MHC class II molecules are generated mostly by degradation of proteins that access the endocytic route, where they are processed by lysosomal proteases and other hydrolases. Exogenous antigens that have been endocytosed by the APC are thus readily available for presentation via MHC II molecules, and for this reason this antigen presentation pathway is usually referred to as exogenous. As membrane proteins on their way to degradation in lysosomes as part of their normal turn-over are also contained in the endosomal/lysosomal compartments, exogenous antigens must compete with those derived from endogenous components. Autophagy is also a source of endogenous peptides, autophagosomes constitutively fuse with MHC class II loading compartments. In addition to APCs, other cells of the gastrointestinal tract, such as epithelial cells, express MHC class II molecules and CD74 and act as APCs, which is an unusual trait of the GI tract. To produce a MHC class II molecule that presents an antigen, three MHC class II molecules (heterodimers of an alpha and a beta chain) associate with a CD74 trimer in the ER to form a heterononamer. Soon after the entry of this complex into the endosomal/lysosomal system where antigen processing occurs, CD74 undergoes a sequential degradation by various proteases, including CTSS and CTSL, leaving a small fragment termed CLIP (class-II-associated invariant chain peptide). The removal of CLIP is facilitated by HLA-DM via direct binding to the alpha-beta-CLIP complex so that CLIP is released. HLA-DM stabilizes MHC class II molecules until primary high affinity antigenic peptides are bound. The MHC II molecule bound to a peptide is then transported to the cell membrane surface. In B-cells, the interaction between HLA-DM and MHC class II molecules is regulated by HLA-DO. Primary dendritic cells (DCs) also to express HLA-DO. Lysosomal miroenvironment has been implicated in the regulation of antigen loading into MHC II molecules, increased acidification produces increased proteolysis and efficient peptide loading. Evolutionary ConservationCheck, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf. Publication Abstract from PubMedCeliac disease, also known as celiac sprue, is a gluten-induced autoimmune-like disorder of the small intestine, which is strongly associated with HLA-DQ2. The structure of DQ2 complexed with an immunogenic epitope from gluten, QLQPFPQPELPY, has been determined to 2.2-A resolution by x-ray crystallography. The glutamate at P6, which is formed by tissue transglutaminase-catalyzed deamidation, is an important anchor residue as it participates in an extensive hydrogen-bonding network involving Lys-beta71 of DQ2. The gluten peptide-DQ2 complex retains critical hydrogen bonds between the MHC and the peptide backbone despite the presence of many proline residues in the peptide that are unable to participate in amide-mediated hydrogen bonds. Positioning of proline residues such that they do not interfere with backbone hydrogen bonding results in a reduction in the number of registers available for gluten peptides to bind to MHC class II molecules and presumably impairs the likelihood of establishing favorable side-chain interactions. The HLA association in celiac disease can be explained by a superior ability of DQ2 to bind the biased repertoire of proline-rich gluten peptides that have survived gastrointestinal digestion and that have been deamidated by tissue transglutaminase. Finally, surface-exposed proline residues in the proteolytically resistant ligand were replaced with functionalized analogs, thereby providing a starting point for the design of orally active agents for blocking gluten-induced toxicity. Structural basis for HLA-DQ2-mediated presentation of gluten epitopes in celiac disease.,Kim CY, Quarsten H, Bergseng E, Khosla C, Sollid LM Proc Natl Acad Sci U S A. 2004 Mar 23;101(12):4175-9. Epub 2004 Mar 12. PMID:15020763[1] From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine. See AlsoReferences
| ||||||||||||||||||
Categories: Human | Large Structures | Bergseng, E | Khosla, C | Kim, C Y | Quarsten, H | Sollid, L M | Hla-dq2 | Immune system